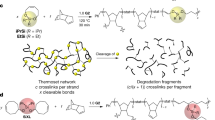
Abstract
Chemical recycling of polymers is critical for improving the circular economy of plastics and environmental sustainability. Traditional thermoset polymers have generally been considered permanently crosslinked materials that are difficult or impossible to recycle. Herein, we demonstrate that by activating ‘dormant’ covalent bonds, traditional polycyanurate thermosets can be recycled into the original monomers, which can be circularly reused for their original purpose. Through retrosynthetic analysis, we redirected the synthetic route from forming conventional C–N bonds via irreversible cyanate trimerization to forming the C–O bonds through reversible nucleophilic aromatic substitution of alkoxy-substituted triazine derivatives by alcohol nucleophiles. The new reversible synthetic route enabled the synthesis of previously inaccessible alkyl-polycyanurate thermosets, which exhibit excellent film properties with high chemical resistance, closed-loop recyclability and reprocessing capability. These results show that ‘apparently dormant’ dynamic linkages can be activated and utilized to construct fully recyclable thermoset polymers with a broader monomer scope and increased sustainability.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Data supporting the findings of this study are included in the Article and its Supplementary Information. Data are also available upon request. Source data are provided with this paper.
References
Geyer, R., Jambeck, J. R. & Law, K. L. Production, use, and fate of all plastics ever made. Sci. Adv. 3, e1700782 (2017).
MacLeod, M., Arp, H. P. H., Tekman, M. B. & Jahnke, A. The global threat from plastic pollution. Science 373, 61–65 (2021).
Garcia, J. M. & Robertson, M. L. The future of plastics recycling. Science 358, 870–872 (2017).
Ragaert, K., Delva, L. & Van Geem, K. Mechanical and chemical recycling of solid plastic waste. Waste Manage. 69, 24–58 (2017).
Eagan, J. M. et al. Combining polyethylene and polypropylene: enhanced performance with PE/iPP multiblock polymers. Science 355, 814–816 (2017).
Coates, G. W. & Getzler, Y. D. Y. L. Chemical recycling to monomer for an ideal, circular polymer economy. Nat. Rev. Mater. 5, 501–516 (2020).
Vollmer, I. et al. Beyond mechanical recycling: giving new life to plastic waste. Angew. Chem. Int. Ed. 59, 15402–15423 (2020).
Korley, L. T. J., Epps, T. H. III, Helms, B. A. & Ryan, A. J. Toward polymer upcycling—adding value and tackling circularity. Science 373, 66–69 (2021).
Zhu, J.-B., Watson, E. M., Tang, J. & Chen, E. Y.-X. A synthetic polymer system with repeatable chemical recyclability. Science 360, 398–403 (2018).
Christensen, P. R., Scheuermann, A. M., Loeffler, K. E. & Helms, B. A. Closed-loop recycling of plastics enabled by dynamic covalent diketoenamine bonds. Nat. Chem. 11, 442–448 (2019).
Abel, B. A., Snyder, R. L. & Coates, G. W. Chemically recyclable thermoplastics from reversible-deactivation polymerization of cyclic acetals. Science 373, 783–789 (2021).
Haussler, M., Eck, M., Rothauer, D. & Mecking, S. Closed-loop recycling of polyethylene-like materials. Nature 590, 423–427 (2021).
Mohadjer Beromi, M. et al. Iron-catalysed synthesis and chemical recycling of telechelic 1,3-enchained oligocyclobutanes. Nat. Chem. 13, 156–162 (2021).
Sathe, D. et al. Olefin metathesis-based chemically recyclable polymers enabled by fused-ring monomers. Nat. Chem. 13, 743–750 (2021).
Shieh, P. et al. Cleavable comonomers enable degradable, recyclable thermoset plastics. Nature 583, 542–547 (2020).
Jin, Y., Yu, C., Denman, R. J. & Zhang, W. Recent advances in dynamic covalent chemistry. Chem. Soc. Rev. 42, 6634–6654 (2013).
Kloxin, C. J. & Bowman, C. N. Covalent adaptable networks: smart, reconfigurable and responsive network systems. Chem. Soc. Rev. 42, 7161–7173 (2013).
Denissen, W., Winne, J. M. & Du Prez, F. E. Vitrimers: permanent organic networks with glass-like fluidity. Chem. Sci. 7, 30–38 (2016).
Fortman, D. J. et al. Approaches to sustainable and continually recyclable cross-linked polymers. ACS Sustain. Chem. Eng. 6, 11145–11159 (2018).
Jin, Y., Lei, Z., Taynton, P., Huang, S. & Zhang, W. Malleable and recyclable thermosets: the next generation of plastics. Matter 1, 1456–1493 (2019).
Garcia, J. M. et al. Recyclable, strong thermosets and organogels via paraformaldehyde condensation with diamines. Science 344, 732–735 (2014).
Taynton, P. et al. Heat- or water-driven malleability in a highly recyclable covalent network polymer. Adv. Mater. 26, 3938–3942 (2014).
Montarnal, D., Capelot, M., Tournilhac, F. & Leibler, L. Silica-like malleable materials from permanent organic networks. Science 334, 965–968 (2011).
Röttger, M. et al. High-performance vitrimers from commodity thermoplastics through dioxaborolane metathesis. Science 356, 62–65 (2017).
Cash, J. J., Kubo, T., Bapat, A. P. & Sumerlin, B. S. Room-temperature self-healing polymers based on dynamic-covalent boronic esters. Macromolecules 48, 2098–2106 (2015).
Denissen, W. et al. Vinylogous urethane vitrimers. Adv. Funct. Mater. 25, 2451–2457 (2015).
Fortman, D. J., Brutman, J. P., Cramer, C. J., Hillmyer, M. A. & Dichtel, W. R. Mechanically activated, catalyst-free polyhydroxyurethane vitrimers. J. Am. Chem. Soc. 137, 14019–14022 (2015).
Nishimura, Y., Chung, J., Muradyan, H. & Guan, Z. Silyl ether as a robust and thermally stable dynamic covalent motif for malleable polymer design. J. Am. Chem. Soc. 139, 14881–14884 (2017).
Yue, L., Amirkhosravi, M., Gong, X., Gray, T. G. & Manas-Zloczower, I. Recycling epoxy by vitrimerization: influence of an initial thermoset chemical structure. ACS Sustain. Chem. Eng. 8, 12706–12712 (2020).
Yang, H., Jin, Y., Du, Y. & Zhang, W. Application of alkyne metathesis in polymer synthesis. J. Mater. Chem. A 2, 5986–5993 (2014).
Gross, D. E., Discekici, E. & Moore, J. S. Macrocyclic depolymerization of arylene-ethynylene copolymers: a dynamic combinatorial method. Chem. Commun. 48, 4426–4428 (2012).
Ramdani, N., Zaimeche, H. & Derradji, M. Biobased thermally-stable aromatic cyanate ester thermosets: a review. React. Funct. Polym. 168, 105037 (2021).
Kandelbauer, A. in Handbook of Thermoset Plastics 3rd edn (eds Dodiuk, H. & Goodman, S. H.) 425–457 (William Andrew Publishing, 2014).
Hamerton, I. Chemistry and Technology of Cyanate Ester Resins (Springer, 1994).
Söthje, D., Dreyer, C. & Bauer, M. Advanced Possibilities in Thermoset Recycling in Thermosets 2013 From Monomers to Components Proceedings of the 3rd International Conference on Thermosets, 18–20 September 2013, Berlin, Germany (Fraunhofer Research Institution for Polymeric Materials and Composites PYCO, 2013).
Söthje, D., Bauer, M. & Dreyer, C. Process for reutilization of recycled polycyanurate-containing materials in new polymeric materials. International patent, EP2885338A1 (2014).
Dreyer, C. & Dominik, S. Progress in recycling of composites with polycyanurate matrix. Adv. Chem. Eng. Sci. 4, 167–183 (2014).
Fainleib, A. M., Hourston, D. J., Grigoryeva, O. P., Shantalii, T. A. & Sergeeva, L. M. Structure development in aromatic polycyanurate networks modified with hydroxyl-terminated polyethers. Polymer 42, 8361–8372 (2001).
Rohrbach, S. et al. Concerted nucleophilic aromatic substitution reactions. Angew. Chem. Int. Ed. 58, 16368–16388 (2019).
Ong, W. J. & Swager, T. M. Dynamic self-correcting nucleophilic aromatic substitution. Nat. Chem. 10, 1023–1030 (2018).
Jin, Y. & Zhang, W. SNAr stands corrected. Nat. Chem. 10, 996–998 (2018).
Armarego, W. L. F. Purification of Laboratory Chemicals 148 (Elsevier Science, 2017).
Acknowledgements
We thank D. Walba for the help with differential scanning calorimetry characterization. We acknowledge Colorado State University Analytical Resources Core (RRID SCR_021758) for gas chromatography–mass spectrometry and thermogravimetric analysis characterizations. K.Y. acknowledges the support from the National Science Foundation (grant CMMI-1901807). W.Z. acknowledges the support from University of Colorado Boulder.
Author information
Authors and Affiliations
Contributions
Z.L. and W.Z. conceived the concept and led the project. Z.L., H.C. and Y.R. performed the small-molecule model study, polymer synthesis, characterization and recycling. C.L. performed the dynamic mechanical analysis. Y.H. performed the differential scanning calorimetry tests. Z.L., Y.J. and W.Z. wrote the manuscript. All authors discussed and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
Z.L. and W.Z. are coinventors on a provisional US patent covering the methods of polymerization and composition of matter presented in this work, filed through the University of Colorado Boulder (application no. 63/354,754). The other authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Small molecule study of cyanurate exchange.
a, No reaction between TETA and methanol was observed without TBD catalyst. b, The first step of exchange reaction between TETA and deuterated methanol can be considered as an irreversible pseudo first-order reaction when the deuterated methanol is used as solvent.
Extended Data Fig. 2 FTIR comparison.
a, FTIR spectra of PCN-A4 film and its corresponding monomers. b, FTIR spectra of PCN-A6 film and its corresponding monomers. c, FTIR spectra of PCN-A12 film and its corresponding monomers.
Extended Data Fig. 3 Chemical resistance test of PCN films.
a, Chemical resistance test for PCN-A4 film. b, Chemical resistance test for PCN-A6 film. c, Chemical resistance test for PCN-A12 film. The PCN films were cut into the rectangular shape and submerged in different solutions (1 M HCl, 1 M NaOH, 30% H2O2 and 1 M NaBH4); the top, middle and bottom photos were taken before submerging, after 48-hour submerging, and after drying, respectively. No change in appearance was observed for all the PCN films.
Extended Data Fig. 4 Chemical recycling of PCN-A12.
a, 1H-NMR spectra show that the film degradation in ethanol is clean (TMB, 1,3,5-trimethoxybenzene, used as internal standard) and the recycled DO-12 and TETA are in high purity. b, Nearly identical loss factors for the original and recycled PCN-A12 samples. c, Nearly identical FTIR spectra of the original and recycled PCN-A12 samples.
Extended Data Fig. 5 Chemical recycling of PCN-A4.
a, 1H-NMR spectra show that the film degradation in ethanol is clean (TMB used as internal standard). and the recycled TETA is in high purity. b, Nearly identical loss factors for the original and recycled PCN-A4 samples. c, Nearly identical FTIR spectra of the original and recycled PCN-A4 samples.
Extended Data Fig. 6 Chemical recycling of PCN-A6.
a, 1H-NMR spectra show that the film degradation in ethanol is clean (TMB used as internal standard) and the recycled TETA is in high purity. b, Neary identical loss factor for the original and recycled PCN-A6 samples. c, Nearly identical FTIR spectra of the original and recycled PCN-A6 samples.
Extended Data Fig. 7 Kinetic study of cyanurate exchange in polymer.
a, Bond exchange reaction can be triggered under heat in the presence of 23 mol% excess of diol monomers and 10 mol% of TBD. b, Stress relaxation test of PCN-A6-m at various temperatures. c, Arrhenius plot and its linear fitting. The activation energy was calculated to be 76.7 kJ/mol.
Supplementary information
Supplementary Information
Supplementary Figs. 1–26, Tables 1–5 and Discussion.
Source data
Source Data Fig. 2
Concentration data points.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lei, Z., Chen, H., Luo, C. et al. Recyclable and malleable thermosets enabled by activating dormant dynamic linkages. Nat. Chem. 14, 1399–1404 (2022). https://doi.org/10.1038/s41557-022-01046-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-022-01046-4
This article is cited by
-
Catalyst-Free and Sustainable Bio-Based Epoxy Vitrimer Prepared Based on Ester Exchange and Imine Bonding
Journal of Polymers and the Environment (2024)
-
Biorenewable and circular polydiketoenamine plastics
Nature Sustainability (2023)
-
Poly(imine-amide) Hybrid Covalent Adaptable Networks via in situ Oxidation Polymerization
Chinese Journal of Polymer Science (2023)