Abstract
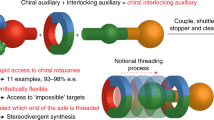
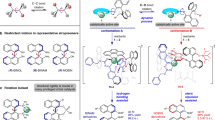
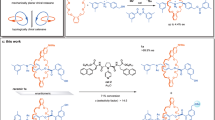
Chirality typically arises in molecules because of a rigidly chiral arrangement of covalently bonded atoms. Less generally appreciated is that chirality can arise when molecules are threaded through one another to create a mechanical bond. For example, when two macrocycles with chemically distinct faces are joined to form a catenane, the structure is chiral, although the rings themselves are not. However, enantiopure mechanically axially chiral catenanes in which the mechanical bond provides the sole source of stereochemistry have not been reported. Here we re-examine the symmetry properties of these molecules and in doing so identify a straightforward route to access them from simple chiral building blocks. Our analysis also led us to identify an analogous but previously unremarked upon rotaxane stereogenic unit, which also yielded to our co-conformational auxiliary approach. With methods to access mechanically axially chiral molecules in hand, their properties and applications can now be explored.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All characterization data for novel compounds (NMR, MS, CD, HPLC) are available through the University of Southampton data repository (https://doi.org/10.5258/SOTON/D2185). Crystallographic data have been uploaded to the CCDC and are available under accession nos. 2109976 (rac-(Sma,Rco-c)-3), 2115463 (rac-6), 2109991 (rac-S15) and 2109992 ((Rma,Rco-c)-9).
References
Kelvin, W. T. Baltimore Lectures on Molecular Dynamics and the Wave Theory of Light 619 (C. J. Clay & Sons, 1904).
Zschiesche, D. et al. Chiral symmetries in nuclear physics. HNPS Adv. Nucl. Phys 9, 170–209 (2020).
Petitjean, M. Symmetry, antisymmetry and chirality: use and misuse of terminology. Symmetry 13, 603 (2021).
Mezey, P. G. Chirality measures and graph representations. Comput. Math. Appl. 34, 105–112 (1997).
da Motta, H. Chirality and neutrinos, a student first approach. J. Phys. Conf. Ser. 1558, 012014 (2020).
Diaz, D. B. et al. Illuminating the dark conformational space of macrocycles using dominant rotors. Nat. Chem. 13, 218–225 (2021).
Eliel, E. L., Wilen, S. H. & Mander, L. N. Stereochemistry of Organic Compounds (Wiley, 1994).
The Nobel Prize in Chemistry 2001; https://www.nobelprize.org
The Nobel Prize in Chemistry 2021; https://www.nobelprize.org
Mislow, K. & Siegel, J. Stereoisomerism and local chirality. J. Am. Chem. Soc. 106, 3319–3328 (1984).
Herges, R. Topology in chemistry: designing Mobius molecules. Chem. Rev. 106, 4820–4842 (2006).
Fielden, S. D. P., Leigh, D. A. & Woltering, S. L. Molecular knots. Angew. Chem. Int. Ed. 56, 11166–11194 (2017).
Bruns, C. J. & Stoddart, J. F. The Nature of the Mechanical Bond: From Molecules to Machines (Wiley, 2016).
Jamieson, E. M. G., Modicom, F. & Goldup, S. M. Chirality in rotaxanes and catenanes. Chem. Soc. Rev. 47, 5266–5311 (2018).
Frisch, H. L. & Wasserman, E. Chemical topology. J. Am. Chem. Soc. 83, 3789–3795 (1961).
Schill, G. Catenanes, Rotaxanes and Knots (Academic Press, 1971).
Maynard, J. R. J. & Goldup, S. M. Strategies for the synthesis of enantiopure mechanically chiral molecules. Chem 6, 1914–1932 (2020).
Chambron, J. C., Dietrich-Buchecker, C., Rapenne, G. & Sauvage, J. P. Resolution of topologically chiral molecular objects. Chirality 10, 125–133 (1998).
Yamamoto, C., Okamoto, Y., Schmidt, T., Jager, R. & Vogtle, F. Enantiomeric resolution of cycloenantiomeric rotaxane, topologically chiral catenane, and pretzel-shaped molecules: observation of pronounced circular dichroism. J. Am. Chem. Soc. 119, 10547–10548 (1997).
Hirose, K. et al. The asymmetry is derived from mechanical interlocking of achiral axle and achiral ring components—syntheses and properties of optically pure [2]rotaxanes. Symmetry 10, 20 (2018).
Gaedke, M. et al. Chiroptical inversion of a planar chiral redox-switchable rotaxane. Chem. Sci. 10, 10003–10009 (2019).
Imayoshi, A. et al. Enantioselective preparation of mechanically planar chiral rotaxanes by kinetic resolution strategy. Nat. Commun. 12, 404 (2021).
Tian, C., Fielden, S. D. P., Perez-Saavedra, B., Vitorica-Yrezabal, I. J. & Leigh, D. A. Single-step enantioselective synthesis of mechanically planar chiral [2]rotaxanes using a chiral leaving group strategy. J. Am. Chem. Soc. 142, 9803–9808 (2020).
Bordoli, R. J. & Goldup, S. M. An efficient approach to mechanically planar chiral rotaxanes. J. Am. Chem. Soc. 136, 4817–4820 (2014).
Jinks, M. A. et al. Stereoselective synthesis of mechanically planar chiral rotaxanes. Angew. Chem. Int. Ed. 57, 14806–14810 (2018).
de Juan, A. et al. A chiral interlocking auxiliary strategy for the synthesis of mechanically planar chiral rotaxanes. Nat. Chem. 14, 179–187 (2022).
Denis, M., Lewis, J. E. M., Modicom, F. & Goldup, S. M. An auxiliary approach for the stereoselective synthesis of topologically chiral catenanes. Chem 5, 1512–1520 (2019).
McArdle, C. P., Van, S., Jennings, M. C. & Puddephatt, R. J. Gold(I) macrocycles and topologically chiral [2]catenanes. J. Am. Chem. Soc. 124, 3959–3965 (2002).
Habermehl, N. C., Jennings, M. C., McArdle, C. P., Mohr, F. & Puddephatt, R. J. Selectivity in the self-assembly of organometallic gold(I) rings and [2]catenanes. Organometallics 24, 5004–5014 (2005).
Theil, A., Mauve, C., Adeline, M.-T., Marinetti, A. & Sauvage, J.-P. Phosphorus-containing [2]catenanes as an example of interlocking chiral structures. Angew. Chem. Int. Ed. 45, 2104–2107 (2006).
IUPAC. Compendium of Chemical Terminology (the ‘Gold Book’) 2nd edn (eds McNaught, A. D. and Wilkinson, A) (Blackwell Scientific Publications, 1997); https://doi.org/10.1351/goldbook
Alvarez-Perez, M., Goldup, S. M., Leigh, D. A. & Slawin, A. M. A chemically-driven molecular information ratchet. J. Am. Chem. Soc. 130, 1836–1838 (2008).
Carlone, A., Goldup, S. M., Lebrasseur, N., Leigh, D. A. & Wilson, A. A three-compartment chemically-driven molecular information ratchet. J. Am. Chem. Soc. 134, 8321–8323 (2012).
Cakmak, Y., Erbas-Cakmak, S. & Leigh, D. A. Asymmetric catalysis with a mechanically point-chiral rotaxane. J. Am. Chem. Soc. 138, 1749–1751 (2016).
Lewis, J. E. M. et al. High yielding synthesis of 2,2′-bipyridine macrocycles, versatile intermediates in the synthesis of rotaxanes. Chem. Sci. 7, 3154–3161 (2016).
Denis, M. & Goldup, S. M. The active template approach to interlocked molecules. Nat. Rev. Chem. 1, 0061 (2017).
Aucagne, V., Hanni, K. D., Leigh, D. A., Lusby, P. J. & Walker, D. B. Catalytic ‘click’ rotaxanes: a substoichiometric metal-template pathway to mechanically interlocked architectures. J. Am. Chem. Soc. 128, 2186–2187 (2006).
Lewis, J. E. M., Modicom, F. & Goldup, S. M. Efficient multicomponent active template synthesis of catenanes. J. Am. Chem. Soc. 140, 4787–4791 (2018).
Shukla, V. G., Salgaonkar, P. D. & Akamanchi, K. G. A mild, chemoselective oxidation of sulfides to sulfoxides using o-iodoxybenzoic acid and tetraethylammonium bromide as catalyst. J. Org. Chem. 68, 5422–5425 (2003).
Lahlali, H., Jobe, K., Watkinson, M. & Goldup, S. M. Macrocycle size matters: ‘small’ functionalized rotaxanes in excellent yield using the CuAAC active template approach. Angew. Chem. Int. Ed. 50, 4151–4155 (2011).
Schroder, H. V., Zhang, Y. & Link, A. J. Dynamic covalent self-assembly of mechanically interlocked molecules solely made from peptides. Nat. Chem 13, 850–857 (2021).
Walba, D. M. Topological stereochemistry. Tetrahedron 41, 3161–3212 (1985).
Canfield, P. J. et al. A new fundamental type of conformational isomerism. Nat. Chem. 10, 615–624 (2018).
Canfield, P. J., Govenlock, L. J., Reimers, J. & Crossley, M. J. Recent advances in stereochemistry reveal classification shortcomings. Preprint at https://doi.org/10.26434/chemrxiv.12488525.v1 (2020).
Reisberg, S. H. et al. Total synthesis reveals atypical atropisomerism in a small-molecule natural product, tryptorubin A. Science 367, 458–463 (2020).
Lukin, O., Godt, A. & Vogtle, F. Residual topological isomerism of intertwined molecules. Chem. Eur. J. 10, 1878–1883 (2004).
Martinez-Cuezva, A., Saura-Sanmartin, A., Alajarin, M. & Berna, J. Mechanically interlocked catalysts for asymmetric synthesis. ACS Catal. 10, 7719–7733 (2020).
Pairault, N. & Niemeyer, J. Chiral mechanically interlocked molecules—applications of rotaxanes, catenanes and molecular knots in stereoselective chemosensing and catalysis. Synlett 29, 689–698 (2018).
Mitra, R., Zhu, H., Grimme, S. & Niemeyer, J. Functional mechanically interlocked molecules: asymmetric organocatalysis with a catenated bifunctional Brønsted acid. Angew. Chem. Int. Ed. 56, 11456–11459 (2017).
Pairault, N. et al. Heterobifunctional rotaxanes for asymmetric catalysis. Angew. Chem. Int. Ed. 59, 5102–5107 (2020).
Dommaschk, M., Echavarren, J., Leigh, D. A., Marcos, V. & Singleton, T. A. Dynamic control of chiral space through local symmetry breaking in a rotaxane organocatalyst. Angew. Chem. Int. Ed. 58, 14955–14958 (2019).
Heard, A. W. & Goldup, S. M. Synthesis of a mechanically planar chiral rotaxane ligand for enantioselective catalysis. Chem 6, 994–1006 (2020).
Gaedke, M. et al. Chiroptical inversion of a planar chiral redox-switchable rotaxane. Chem. Sci. 10, 10003–10009 (2019).
Corra, S. et al. Chemical on/off switching of mechanically planar chirality and chiral anion recognition in a [2]rotaxane molecular shuttle. J. Am. Chem. Soc. 141, 9129–9133 (2019).
Wood, C. S., Ronson, T. K., Belenguer, A. M., Holstein, J. J. & Nitschke, J. R. Two-stage directed self-assembly of a cyclic [3]catenane. Nat. Chem. 7, 354–358 (2015).
Caprice, K. et al. Diastereoselective amplification of a mechanically chiral [2]catenane. J. Am. Chem. Soc. 143, 11957–11962 (2021).
Cui, Z., Lu, Y., Gao, X., Feng, H. J. & Jin, G. X. Stereoselective synthesis of a topologically chiral Solomon link. J. Am. Chem. Soc. 142, 13667–13671 (2020).
Carpenter, J. P. et al. Controlling the shape and chirality of an eight-crossing molecular knot. Chem 7, 1534–1543 (2021).
Leigh, D. A. et al. Tying different knots in a molecular strand. Nature 584, 562–568 (2020).
Corra, S., de Vet, C., Baroncini, M., Credi, A. & Silvi, S. Stereodynamics of E/Z isomerization in rotaxanes through mechanical shuttling and covalent bond rotation. Chem 7, 2137–2150 (2021).
David, A. H. G. & Stoddart, J. F. Chiroptical properties of mechanically interlocked molecules. Isr. J. Chem. 61, 608–621 (2021).
Ashton, P. R. et al. Molecular meccano, part 23. Self-assembling cyclophanes and catenanes possessing elements of planar chirality. Chem. A Eur. J. 4, 299–310 (1998).
Caprice, K. et al. Diastereoselective amplification of a mechanically chiral [2]catenane. J. Am. Chem. Soc. 143, 11957–11962 (2021).
Acknowledgements
S.M.G. thanks the ERC (agreement no. 724987) and the Royal Society for a Wolfson Research Fellowship (RSWF\FT\180010). P.B. thanks the University of Southampton for a Presidential Scholarship. P.G. thanks the University of Southampton for funding.
Author information
Authors and Affiliations
Contributions
J.R.J.M. and P.G. contributed equally. Both have the right to place themselves as first author on their CVs. J.R.J.M. and S.M.G. developed the co-conformational auxiliary concept. J.R.J.M. synthesized 3 and 5 and collected SCXRD diffraction data for a reduced product of catenane 5. P.G. synthesized 9 and 10, determined the stereochemistry of rotaxanes 9, and managed the preparation of manuscript graphics. D.L. optimized the synthesis and purification of 3 and 5, synthesized 6 and determined the stereochemistry of catenanes 3. P.B. collected the X-ray diffraction data of 3, 6 and 9 and fully refined all SCXRD data. D.L. and P.G. managed the preparation of the Supplementary Information. S.M.G. directed the research. All authors contributed to the analysis of the results and the writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Experimental procedures and analytical data for compounds 1–10 and S1–S21 and elaborated discussions of manuscript content.
Supplementary Data 1
Crystallographic data for catenane rac-(Sma,Rco-c)-3; (CCDC reference, 2109976)
Supplementary Data 2
Crystallographic data for catenane rac-6; (CCDC reference, 2115463)
Supplementary Data 3
Crystallographic data for catenane rac-S15; (CCDC reference, 2109991)
Supplementary Data 4
Crystallographic data for rotaxane (Rma,Rco-c)-9; (CCDC reference, 2109992)
Rights and permissions
About this article
Cite this article
Maynard, J.R.J., Gallagher, P., Lozano, D. et al. Mechanically axially chiral catenanes and noncanonical mechanically axially chiral rotaxanes. Nat. Chem. 14, 1038–1044 (2022). https://doi.org/10.1038/s41557-022-00973-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-022-00973-6
This article is cited by
-
A catenane that is topologically achiral despite being composed of oriented rings
Nature Chemistry (2023)