Abstract
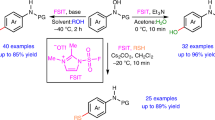
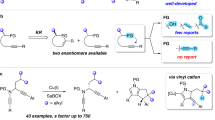
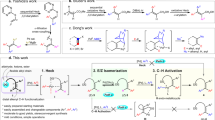
Over the past three decades, organocatalysis has emerged as a powerful catalysis platform and has gradually been incorporated into the routine synthetic toolbox to obtain chiral molecules. However, its application in the site- and enantioselective functionalization of inactive aryl C–H bonds remains in its infancy. Here, we present an organocatalyst-controlled para-selective arene C–H functionalization strategy that addresses this issue, which remains an enduring challenge in arene functionalization chemistry. By emulating enzyme catalysis, the chiral phosphoric acid catalyst offers an ideal chiral environment for stereoinduction, and the projecting substituents give control of chemo- and site-selectivity. Various types of nucleophile are compatible with this method, affording more than 100 para-selective adducts with stereodefined carbon centres or axes in viable molecular contexts. This protocol is expected to provide a general strategy for para-selective functionalization of arene C–H bonds in a controlled manner.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The X-ray crystallographic coordinates for the products reported in this Article have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition nos. CCDC 1992074 (12j), CCDC 1992075 (12r), CCDC 1992076 (12s), CCDC 1992068 (14), 1992069 (16) and 1992073 (18). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/. Experimental procedures and characterization of new compounds are available in the Supplementary Information.
References
Abrams, D. J., Provencher, P. A. & Sorensen, E. J. Recent applications of C–H functionalization in complex natural product synthesis. Chem. Soc. Rev. 47, 8925–8967 (2018).
Wencel-Delord, J. & Glorius, F. C–H bond activation enables the rapid construction and late-stage diversification of functional molecules. Nat. Chem. 5, 369–375 (2013).
Yamaguchi, J., Yamaguchi, A. D. & Itami, K. C–H bond functionalization: emerging synthetic tools for natural products and pharmaceuticals. Angew. Chem. Int. Ed. 51, 8960–9009 (2012).
Zhou, Z. & Parr, R. G. Activation hardness: new index for describing the orientation of electrophilic aromatic substitution. J. Am. Chem. Soc. 112, 5720–5724 (1990).
Dey, A., Maity, S. & Maiti, D. Reaching the south: metal-catalyzed transformation of the aromatic para-position. Chem. Commun. 52, 12398–12414 (2016).
Romero, N. A., Margrey, K. A., Tay, N. E. & Nicewicz, D. A. Site-selective arene C–H amination via photoredox catalysis. Science 349, 1326–1330 (2015).
Boursalian, G. B., Ham, W. S., Mazzotti, A. R. & Ritter, T. Charge-transfer-directed radical substitution enables para-selective C–H functionalization. Nat. Chem. 8, 810–815 (2016).
Berger, F. et al. Site-selective and versatile aromatic C−H functionalization by thianthrenation. Nature 567, 223–228 (2019).
Bag, S. et al. Remote para-C–H functionalization of arenes by a D-shaped biphenyl template-based assembly. J. Am. Chem. Soc. 137, 11888–11891 (2015).
Maji, A. et al. Experimental and computational exploration of para-selective silylation with a hydrogen-bonded template. Angew. Chem. Int. Ed. 56, 14903–14907 (2017).
Patra, T. et al. Palladium-catalyzed directed para C−H functionalization of phenols. Angew. Chem. Int. Ed. 55, 7751–7755 (2016).
Hoque, M. E., Bisht, R., Haldar, C. & Chattopadhyay, B. Noncovalent interactions in Ir-catalyzed C–H activation: L-shaped ligand for para-selective borylation of aromatic esters. J. Am. Chem. Soc. 139, 7745–7748 (2017).
Maji, A. et al. H-bonded reusable template assisted para-selective ketonisation using soft electrophilic vinyl ethers. Nat. Commun. 9, 3582 (2018).
Dey, A., Sinha, S. K., Achar, T. K. & Maiti, D. Accessing remote meta- and para-C(sp2)–H bonds with covalently attached directing groups. Angew. Chem. Int. Ed. 58, 10820–10843 (2019).
Dutta, U. et al. Rhodium catalyzed template-assisted distal para-C–H olefination. Chem. Sci. 10, 7426–7432 (2019).
Pimparkar, S. et al. Para-selective cyanation of arenes by H-bonded template. Chem. Eur. J. 26, 11558–11564 (2020).
Shi, H. et al. Differentiation and functionalization of remote C–H bonds in adjacent positions. Nat. Chem. 12, 399–404 (2020).
Rittle, J. & Green, M. T. Cytochrome P450 compound I: capture, characterization and C–H bond activation kinetics. Science 330, 933–937 (2010).
Fishman, A., Tao, Y., Rui, L. & Wood, T. K. Controlling the regiospecific oxidation of aromatics via active site engineering of toluene para-monooxygenase of Ralstonia pickettii PKO1. J. Biol. Chem. 280, 506–514 (2005).
Liao, K., Negretti, S., Musaev, D. G., Bacsa, J. & Davies, H. M. L. Site-selective and stereoselective functionalization of unactivated C–H bonds. Nature 533, 230–234 (2016).
Liao, K. et al. Site-selective and stereoselective functionalization of non-activated tertiary C–H bonds. Nature 551, 609–613 (2017).
Liao, K. et al. Design of catalysts for site-selective and enantioselective functionalization of non-activated primary C–H bonds. Nat. Chem. 10, 1048–1055 (2018).
Cheng, C. & Hartwig, J. F. Rhodium-catalyzed intermolecular C–H silylation of arenes with high steric regiocontrol. Science 343, 853–857 (2014).
Ma, B. et al. Highly para-selective C−H alkylation of benzene derivatives with 2,2,2-trifluoroethyl α-aryl-α-diazoesters. Angew. Chem. Int. Ed. 56, 2749–2753 (2017).
Okumura, S. et al. para-Selective alkylation of benzamides and aromatic ketones by cooperative nickel/aluminum catalysis. J. Am. Chem. Soc. 138, 14699–14704 (2016).
Saito, Y., Segawa, Y. & Itami, K. para-C–H borylation of benzene derivatives by a bulky iridium catalyst. J. Am. Chem. Soc. 137, 5193–5198 (2015).
Yang, L., Semba, K. & Nakao, Y. para-Selective C−H borylation of (hetero)arenes by cooperative iridium/aluminum catalysis. Angew. Chem. Int. Ed. 56, 4853–4857 (2017).
List, B., Lerner, R. A. & Barbas, C. F. III Proline-catalyzed direct asymmetric aldol reactions. J. Am. Chem. Soc. 122, 2395–2396 (2000).
Ahrendt, K. A., Borths, C. J. & MacMillan, D. W. C. New strategies for organic synthesis: the first highly enantioselective organocatalytic Diels–Alder reaction. J. Am. Chem. Soc. 122, 4243–4244 (2000).
Taylor, M. S. & Jacobsen, E. N. Asymmetric catalysis by chiral hydrogen-bond donors. Angew. Chem. Int. Ed. 45, 1520–1543 (2006).
Mukherjee, S., Yang, J. W., Hoffmann, S. & List, B. Asymmetric enamine catalysis. Chem. Rev. 107, 5471–5569 (2007).
MacMillan, D. W. C. The advent and development of organocatalysis. Nature 455, 304–308 (2008).
Silvi, M. & Melchiorre, P. Enhancing the potential of enantioselective organocatalysis with light. Nature 554, 41–49 (2018).
Metrano, A. J. & Miller, S. J. Peptide-based catalysts reach the outer sphere through remote desymmetrization and atroposelectivity. Acc. Chem. Res. 52, 199–215 (2019).
Chen, J. et al. Carbonyl catalysis enables a biomimetic asymmetric Mannich reaction. Science 360, 1438–1442 (2018).
Tsuji, N. et al. Activation of olefins via asymmetric Brønsted acid catalysis. Science 359, 1501–1505 (2018).
Pupo, G. et al. Asymmetric nucleophilic fluorination under hydrogen bonding phase-transfer catalysis. Science 360, 638–642 (2018).
Gustafson, J. L., Lim, D. & Miller, S. J. Dynamic kinetic resolution of biaryl atropisomers via peptide-catalyzed asymmetric bromination. Science 328, 1251–1255 (2010).
Lin, S. & Jacobsen, E. N. Thiourea-catalysed ring opening of episulfonium ions with indole derivatives by means of stabilizing non-covalent interactions. Nat. Chem. 4, 817–824 (2012).
Knowles, R. R. & Jacobsen, E. N. Attractive noncovalent interactions in asymmetric catalysis: links between enzymes and small molecule catalysts. Proc. Natl Acad. Sci. USA 107, 20678–20685 (2010).
Jung, D., Jang, S. H., Yim, T. & Kim, J. Oxidation potential tunable organic molecules and their catalytic application to aerobic dehydrogenation of tetrahydroquinolines. Org. Lett. 20, 6436–6439 (2018).
Akiyama, T., Itoh, J., Yokota, K. & Fuchibe, K. Enantioselective Mannich-type reaction catalyzed by a chiral Brønsted acid. Angew. Chem. Int. Ed. 43, 1566–1568 (2004).
Uraguchi, D. & Terada, M. Chiral Brønsted acid-catalyzed direct Mannich reactions via electrophilic activation. J. Am. Chem. Soc. 126, 5356–5357 (2004).
Akiyama, T. Stronger Brønsted acids. Chem. Rev. 107, 5744–5758 (2007).
Parmar, D., Sugiono, E., Raja, S. & Rueping, M. Complete field guide to asymmetric BINOL-phosphate derived Brønsted acid and metal catalysis: history and classification by mode of activation; Brønsted acidity, hydrogen bonding, ion pairing and metal phosphates. Chem. Rev. 114, 9047–9153 (2014).
Hansch, C., Leo, A. & Taft, R. W. A survey of Hammett substituent constants and resonance and field parameters. Chem. Rev. 91, 165–195 (1991).
Chen, M. & Sun, J. Catalytic asymmetric N-alkylation of indoles and carbazoles through 1,6-conjugate addition of aza-para-quinone methides. Angew. Chem. Int. Ed. 56, 4583–4587 (2017).
Ma, D., Miao, C.-B. & Sun, J. Catalytic enantioselective House–Meinwald rearrangement: efficient construction of all-carbon quaternary stereocenters. J. Am. Chem. Soc. 141, 13783–13787 (2019).
Uyeda, C. & Jacobsen, E. N. Transition state charge stabilization through multiple non-covalent interactions in the guanidinium-catalyzed enantioselective Claisen rearrangement. J. Am. Chem. Soc. 133, 5062–5075 (2011).
Li, G.-Q. et al. Organocatalytic aryl–aryl bond formation: an atroposelective [3,3]-rearrangement approach to BINAM derivatives. J. Am. Chem. Soc. 135, 7414–7417 (2013).
Qi, L.-W., Mao, J.-H., Zhang, J. & Tan, B. Organocatalytic asymmetric arylation of indoles enabled by azo groups. Nat. Chem. 10, 58–64 (2017).
Acknowledgements
We are grateful for financial support from the National Natural Science Foundation of China (grants 21825105 to B.T. and 21801121 to Y.-B.W.), Guangdong Provincial Key Laboratory of Catalysis (grant 2020B121201002 to B.T.), Guangdong Innovative Program (grant 2019BT02Y335 to B.T.), Shenzhen Special Funds (grants JCYJ20180305123508258 to S.-H.X. and JCYJ20180302174106405 to Y.-B.W.), Shenzhen Nobel Prize Scientists Laboratory Project and SUSTech Special Fund for the Construction of High-Level Universities (grant G02216402, B.T.). We dedicate this paper to the 100th Anniversary of Xiamen University.
Author information
Authors and Affiliations
Contributions
B.T. conceived and directed the project. J.-H.M. and Y.-B.W. designed and performed experiments. L.Y. performed the density functional theory calculations and mechanism analysis. S.-H.X., S.L., Q.-H.W., Y.C., Q.L. and J.L. helped with the collection of some new compounds and data analysis. B.T., S.-H.X., Y.-B.W., S.L. and L.Y. wrote the manuscript with input from all other authors. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Chemistry thanks Debabrata Maiti and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Tables 1–3, Figs. 1–14, experimental data, synthesis and characterization data, NMR spectra, X-ray crystallographic data and density functional theory calculation data.
Supplementary Data 1
Crystallographic data for compound 12j. CCDC reference 1992074.
Supplementary Data 2
Structure factors file for compound 12j. CCDC reference 1992074.
Supplementary Data 3
Crystallographic data for compound 12r. CCDC reference 1992075.
Supplementary Data 4
Structure factors file for compound 12r. CCDC reference 1992075.
Supplementary Data 5
Crystallographic data for compound 12s. CCDC reference 1992076.
Supplementary Data 6
Structure factors file for compound 12s. CCDC reference 1992076.
Supplementary Data 7
Crystallographic data for compound 14. CCDC reference 1992068.
Supplementary Data 8
Structure factors file for compound 14. CCDC reference 1992068.
Supplementary Data 9
Crystallographic data for compound 16. CCDC reference 1992069.
Supplementary Data 10
Structure factors file for compound 16. CCDC reference 1992069.
Supplementary Data 11
Crystallographic data for compound 18. CCDC reference 1992073.
Supplementary Data 12
Structure factors file for compound 18. CCDC reference 1992073.
Rights and permissions
About this article
Cite this article
Mao, JH., Wang, YB., Yang, L. et al. Organocatalyst-controlled site-selective arene C–H functionalization. Nat. Chem. 13, 982–991 (2021). https://doi.org/10.1038/s41557-021-00750-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-021-00750-x
This article is cited by
-
Organocatalytic olefin C–H functionalization for enantioselective synthesis of atropisomeric 1,3-dienes
Nature Catalysis (2024)
-
Ni-catalysed assembly of axially chiral alkenes from alkynyl tetracoordinate borons via 1,3-metallate shift
Nature Chemistry (2024)
-
Regioselective umpolung para-C–H functionalization of arylhydroxylamines
Nature Synthesis (2023)
-
Organocatalytic cycloaddition of alkynylindoles with azonaphthalenes for atroposelective construction of indole-based biaryls
Nature Communications (2022)
-
Design and catalytic atroposelective synthesis of axially chiral isochromenone-indoles
Science China Chemistry (2022)