Abstact
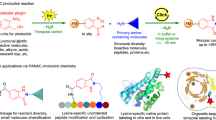
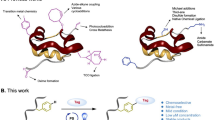
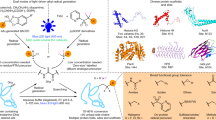
The growing prevalence of synthetically modified proteins in pharmaceuticals and materials has exposed the need for efficient strategies to enable chemical modifications with high site-selectivity. While genetic engineering can incorporate non-natural amino acids into recombinant proteins, regioselective chemical modification of wild-type proteins remains a challenge. Herein, we use photoredox catalysis to develop a site-selective tyrosine bioconjugation pathway that incorporates bioorthogonal formyl groups, which subsequently allows for the synthesis of structurally defined fluorescent conjugates from native proteins. A water-soluble photocatalyst, lumiflavin, has been shown to induce oxidative coupling between a previously unreported phenoxazine dialdehyde tag and a single tyrosine site, even in the presence of multiple tyrosyl side chains, through the formation of a covalent C–N bond. A variety of native proteins, including those with multiple tyrosines, can successfully undergo both tyrosine-specific and single-site-selective labelling. This technology directly introduces aldehyde moieties onto native proteins, enabling rapid product diversification using an array of well-established bioorthogonal functionalization protocols including the alkyne–azide click reaction.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within the article and its Supplementary Information.
References
Stephanopoulos, N. & Francis, M. B. Choosing an effective protein bioconjugation strategy. Nat. Chem. Biol. 7, 876–884 (2011).
Spicer, C. D. & Davis, B. G. Selective chemical protein modification. Nat. Commun. 5, 4740 (2014).
Agarwal, P., Beahm, B. J., Shieh, P. & Bertozzi, C. R. Systemic fluorescence imaging of zebrafish glycans with bioorthogonal chemistry. Angew. Chem. Int. Ed. 54, 11504–11510 (2015).
Hoyt, E. A., Cal, P. M. S. D., Oliveira, B. L. & Bernardes, G. J. L. Contemporary approaches to site-selective protein modification. Nat. Rev. Chem. 3, 147–171 (2019).
deGruter, J. N., Malins, L. R. & Baran, P. S. Residue-specific peptide modification: a chemist’s guide. Biochemistry 56, 3863–3873 (2017).
Boutureira, O. & Bernardes, G. J. L. Advances in chemical protein modification. Chem. Rev. 115, 2174–2195 (2015).
Koniev, O. & Wagner, A. Developments and recent advancements in the field of endogenous amino acid selective bond forming reactions for bioconjugation. Chem. Soc. Rev. 44, 5495–5551 (2015).
Hermanson, G. T. Bioconjugate Techniques (Academic Press, 1996).
Gunnoo, S. B. & Madder, A. Chemical protein modification through cysteine. ChemBioChem 17, 529–553 (2016).
Rosen, C. B. & Francis, M. B. Targeting the N terminus for site-selective protein modification. Nat. Chem. Biol. 13, 697–705 (2017).
Bloom, S. et al. Decarboxylative alkylation for site-selective bioconjugation of native proteins via oxidation potentials. Nat. Chem. 10, 205–211 (2018).
Garreau, M., Le Vaillant, F. & Waser, J. C-terminal bioconjugation of peptides through photoredox catalyzed decarboxylative alkynylation. Angew. Chem. Int. Ed. 58, 8182–8186 (2019).
Joshi, N. S., Whitaker, L. R. & Francis, M. B. A three-component Mannich-type reaction for selective tyrosine bioconjugation. J. Am. Chem. Soc. 126, 15942–15943 (2004).
Ban, H. et al. Facile and stabile linkages through tyrosine: bioconjugation strategies with the tyrosine-click reaction. Bioconjugate Chem. 24, 520–532 (2013).
Alvarez-Dorta, D. et al. Electrochemically promoted tyrosine-click-chemistry for protein labeling. J. Am. Chem. Soc. 140, 17120–17126 (2018).
Song, C. et al. Electrochemical oxidation induced selective tyrosine bioconjugation for the modification of biomolecules. Chem. Sci. 10, 7982–7987 (2019).
Sato, S. & Nakamura, H. Ligand-directed selective protein modification based on local single-electron-transfer catalysis. Angew. Chem. Int. Ed. 52, 8681–8684 (2013).
Seki, Y. et al. Transition metal-free tryptophan-selective bioconjugation of proteins. J. Am. Chem. Soc. 138, 10798–10801 (2016).
Lin, S. et al. Redox-based reagents for chemoselective methionine bioconjugation. Science 355, 597–602 (2017).
Taylor, M. T., Nelson, J. E., Suero, M. G. & Gaunt, M. J. A protein functionalization platform based on selective reactions at methionine residues. Nature 562, 563–568 (2018).
Shaw, M. H., Twilton, J. & MacMillan, D. W. C. Photoredox catalysis in organic chemistry. J. Org. Chem. 81, 6898–6926 (2016).
Bottecchia, C. & Noël, T. Photocatalytic modification of amino acids, peptides, and proteins. Chem. Eur. J. 25, 26–42 (2019).
McCarver, S. J. et al. Decarboxylative peptide macrocyclization through photoredox catalysis. Angew. Chem. Int. Ed. 56, 728–732 (2017).
Kölmel, D. K. et al. On-DNA decarboxylative arylation: merging photoredox with nickel catalysis in water. ACS Comb. Sci. 21, 588–597 (2019).
Montanari, E. et al. Tyrosinase-mediated bioconjugation. A versatile approach to chimeric macromolecules. Bioconjugate Chem. 29, 2550–2560 (2018).
Echols, N. et al. Comprehensive analysis of amino acid and nucleotide composition in eukaryotic genomes, comparing genes and pseudogenes. Nucleic Acids Res. 30, 2515–2523 (2002).
Malik, N. A. & Ali, A. Interaction, thermodynamic, and solubilisation study of amino acid-tyrosine in aqueous anionic and cationic amphiphiles: electrical conductance and spectroscopic studies. Phys. Chem. Liq. 56, 69–79 (2017).
Ruffoni, A. et al. Practical and regioselective amination of arenes using alkyl amines. Nat. Chem. 11, 426–433 (2019).
Berger, F. et al. Site-selective and versatile aromatic C−H functionalization by thianthrenation. Nature 567, 223–228 (2019).
Ham, W. S., Hillenbrand, J., Jacq, J., Genicot, C. & Ritter, T. Divergent late-stage (hetero)aryl C–H amination by the pyridinium radical cation. Angew. Chem. Int. Ed. 58, 532–536 (2019).
Weinberg, D. R. et al. Proton-coupled electron transfer. Chem. Rev. 112, 4016–4093 (2012).
Mantsch, H. H. π-Electronic structure and reactivity of phenoxazine (1), phenothiazine (2), and phenoxthiin (3). Can. J. Chem. 47, 3174–3178 (1969).
Patureau, F. W. The phenol-phenothiazine coupling: an oxidative click concept. ChemCatChem 11, 5227–5231 (2019).
Walling, C. Free Radicals in Solution (John Wiley & Sons, 1957).
Roberts, B. P. Polarity-reversal catalysis of hydrogen-atom abstraction reactions: concepts and applications in organic chemistry. Chem. Soc. Rev. 28, 25–35 (1999).
Penzkofer, A. Photoluminescence behavior of riboflavin and lumiflavin in liquid solutions and solid films. Chem. Phys. 400, 142–153 (2012).
Heelis, P. F. The photophysical and photochemical properties of flavins (isoalloxazines). Chem. Soc. Rev. 1, 15–39 (1982).
Minamihata, K., Goto, M. & Kamiya, N. Control of a tyrosyl radical mediated protein cross-linking reaction by electrostatic interaction. Bioconjugate Chem. 23, 1600–1609 (2012).
Rabuka, D., Rush, J. S., deHart, G. W., Wu, P. & Bertozzi, C. R. Site-specific chemical protein conjugation using genetically encoded aldehyde tags. Nat. Protoc. 5, 1052–1067 (2012).
Crisalli, P. & Kool, E. T. Water-soluble organocatalysts for hydrazone and oxime formation. J. Org. Chem. 78, 1184–1189 (2013).
Whitmore, L. & Wallace, B. A. Protein secondary structure analyses from circular dichroism spectroscopy: methods and reference databases. Biopolymers 89, 392–400 (2008).
Fernández-Suárez, M. & Ting, A. Y. Fluorescent probes for super-resolution imagine in living cell. Nat. Rev. Mol. Cell Biol. 9, 929–943 (2008).
Rodriguez, E. A. et al. The growing and glowing toolbox of fluorescent and photoactive proteins. Trends Biochem. Sci. 42, 111–129 (2017).
Berman, H. M. et al. The protein data bank. Nucleic Acids Res. 28, 235–242 (2000).
Acknowledgements
Research was primarily supported as a part of BioLEC, an Energy Frontier Research Center funded by the U.S. Department of Energy, Office of Science, Basic Energy Sciences under award DE-SC0019370 for all research efforts directed towards bioconjugation studies and spectroscopic analyses. We acknowledge K. Saw, H.H. Shwe and the use of Princeton’s Imaging and Analysis Center, which is partially supported by the Princeton Center for Complex Materials, a National Science Foundation/Materials Research Science and Engineering Centers programme (DMR-1420541). We also acknowledge V. G. Vendavasi and the use of Princeton’s Biophysics Core Facility. We thank T. W. Muir and members of the Muir Laboratory for their advice and analytical support. Finally, we thank T.F. Brewer for additional discussions and advice.
Author information
Authors and Affiliations
Contributions
B.X.L., D.K.K. and S.B. performed and analysed the bioconjugation experiments. R.Y.-C.H. performed and analysed the mass spectrometry experiments. D.G.O. performed and analysed the transient absorption spectroscopy experiments. D.K.K., B.X.L., S.B. and D.W.C.M. designed the experiments. D.K.K., B.X.L. and D.W.C.M. prepared this manuscript. G.D.S., J.X.Q. and W.R.E. provided helpful discussions.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Chemistry thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Experimental details, intact mass spectrometry product characterization, fluorescence assay details, reagent synthesis procedures, NMR spectra, LC-MS/MS spectra and ultrafast spectroscopy details.
Rights and permissions
About this article
Cite this article
Li, B.X., Kim, D.K., Bloom, S. et al. Site-selective tyrosine bioconjugation via photoredox catalysis for native-to-bioorthogonal protein transformation. Nat. Chem. 13, 902–908 (2021). https://doi.org/10.1038/s41557-021-00733-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-021-00733-y
This article is cited by
-
Chemoselective umpolung of thiols to episulfoniums for cysteine bioconjugation
Nature Chemistry (2024)
-
Self-propelled assembly of nanoparticles with self-catalytic regulation for tumour-specific imaging and therapy
Nature Communications (2024)
-
Recent advances in chemical protein synthesis: method developments and biological applications
Science China Chemistry (2024)
-
Posttranslational, site-directed photochemical fluorine editing of protein sidechains to probe residue oxidation state via 19F-nuclear magnetic resonance
Nature Protocols (2023)
-
Quick photofabrication of functional nanospheres from de novo designed peptides for NIR fluorescence and MR imaging
Nano Research (2023)