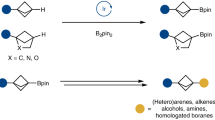
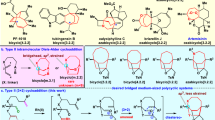
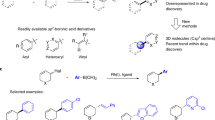
Abstract
Stereodefined four-membered rings are common motifs in bioactive molecules and versatile intermediates in organic synthesis. However, the synthesis of complex, chiral cyclobutanes is a largely unsolved problem and there is a need for general and modular synthetic methods. Here we report a series of asymmetric cross-coupling reactions between cyclobutenes and arylboronic acids which are initiated by Rh-catalysed asymmetric carbometallation. After the initial carborhodation, Rh–cyclobutyl intermediates undergo chain-walking or C–H insertion so that overall a variety of additions such as reductive Heck reactions, 1,5-addition and homoallylic substitution are observed. The synthetic applicability of these highly stereoselective transformations is demonstrated in the concise syntheses of the drug candidates Belaperidone and PF-04862853. We anticipate this approach will be widely adopted by synthetic and medicinal chemists. While the carbometallation approach reported here is exemplified with Rh and arylboronic acids, it is likely to be applicable to other metals and nucleophiles.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data (experimental procedures and characterization data) supporting the findings of this study are available within the article and its Supplementary Information.
References
Lee-Ruff, E. & Mladenova, G. Enantiomerically pure cyclobutane derivatives and their use in organic synthesis. Chem. Rev. 103, 1449–1483 (2003).
Wang, M. & Lu, P. Catalytic approaches to assemble cyclobutane motifs in natural product synthesis. Org. Chem. Front. 5, 254–259 (2018).
Dembitsky, V. M. Naturally occurring bioactive cyclobutane-containing (CBC) alkaloids in fungi, fungal endophytes, and plants. Phytomedicine 21, 1559–1581 (2014).
Namyslo, J. C. & Kaufmann, D. E. The application of cyclobutane derivatives in organic synthesis. Chem. Rev. 103, 1485–1537 (2003).
Seiser, T., Saget, T., Tran, D. N. & Cramer, N. Cyclobutanes in catalysis. Angew. Chem. Int. Ed. 50, 7740–7752 (2011).
Xu, Y., Conner, M. L. & Brown, M. K. Cyclobutane and cyclobutene synthesis: Catalytic enantioselective [2+2] cycloadditions. Angew. Chem. Int. Ed. 54, 11918–11928 (2015).
Pagar, V. V. & RajanBabu, T. V. Tandem catalysis for asymmetric coupling of ethylene and enynes to functionalized cyclobutanes. Science 361, 68–72 (2018).
Brimioulle, R. & Bach, T. Enantioselective lewis acid catalysis of intramolecular enone [2+2] photocycloaddition reactions. Science 342, 840–843 (2013).
Du, J., Skubi, K. L., Schultz, D. M. & Yoon, T. P. A dual-catalysis approach to enantioselective [2 + 2] photocycloadditions using visible light. Science 344, 392–396 (2014).
García-Morales, C. et al. Enantioselective synthesis of cyclobutenes by intermolecular [2+2] cycloaddition with non-C2 symmetric digold catalysts. J. Am. Chem. Soc. 139, 13628–13631 (2017).
Wang, Y. M., Bruno, N. C., Placeres, Á. L., Zhu, S. & Buchwald, S. L. Enantioselective synthesis of carbo- and heterocycles through a CuH-catalyzed hydroalkylation approach. J. Am. Chem. Soc. 137, 10524–10527 (2015).
Kim, D. K., Riedel, J., Kim, R. S. & Dong, V. M. Cobalt catalysis for enantioselective cyclobutanone construction. J. Am. Chem. Soc. 139, 10208–10211 (2017).
Xiao, K. J. et al. Palladium(ii)-catalyzed enantioselective C(sp3)-H activation using a chiral hydroxamic acid ligand. J. Am. Chem. Soc. 136, 8138–8142 (2014).
Zhong, C. et al. Enantioselective synthesis of 3‐substituted cyclobutenes by catalytic conjugate addition/trapping strategies. Angew. Chem. Int. Ed. 59, 2750–2754 (2020).
Chen, Y. J., Hu, T. J., Feng, C. G. & Lin, G. Q. Synthesis of chiral cyclobutanes via rhodium/diene-catalyzed asymmetric 1,4-addition: a dramatic ligand effect on the diastereoselectivity. Chem. Commun. 51, 8773–8776 (2015).
Audisio, D. et al. Palladium-catalyzed allylic substitution at four-membered-ring systems: formation of η1-allyl complexes and electrocyclic ring opening. Angew. Chem. Int. Ed. 52, 6313–6316 (2013).
Guisán-Ceinos, M., Parra, A., Martín-Heras, V. & Tortosa, M. Enantioselective synthesis of cyclobutylboronates via a copper-catalyzed desymmetrization approach. Angew. Chem. Int. Ed. 55, 6969–6972 (2016).
Feng, S., Hao, H., Liu, P. & Buchwald, S. L. Diastereo- and enantioselective CuH-catalyzed hydroamination of strained trisubstituted alkenes. ACS Catal. 10, 282–291 (2020).
Roy, S. R., Eijsberg, H., Bruffaerts, J. & Marek, I. Zirconocene catalyzed diastereoselective carbometalation of cyclobutenes. Chem. Sci. 8, 334–339 (2016).
Didier, D. et al. Modulable and highly diastereoselective carbometalations of cyclopropenes. Chem. Eur. J. 20, 1038–1048 (2014).
Takaya, Y., Ogasawara, M., Hayashi, T., Sakai, M. & Miyaura, N. Rhodium-catalyzed asymmetric 1,4-addition of aryl- and alkenylboronic acids to enones. J. Am. Chem. Soc. 120, 5579–5580 (1998).
Tian, P., Dong, H.-Q. & Lin, G.-Q. Rhodium-catalyzed asymmetric arylation. ACS Catal. 2, 95–119 (2012).
Sidera, M. & Fletcher, S. P. Rhodium-catalysed asymmetric allylic arylation of racemic halides with arylboronic acids. Nat. Chem. 7, 935–939 (2015).
Goetzke, F. W., Mortimore, M. & Fletcher, S. P. Enantio- and diastereoselective Suzuki–Miyaura coupling with racemic bicycles. Angew. Chem. Int. Ed. 58, 12128–12132 (2019).
Hall, D. G. Boronic Acids: Preparation, Applications in Organic Synthesis and Medicine (John Wiley & Sons, 2006).
Brown, D. G. & Boström, J. Analysis of past and present synthetic methodologies on medicinal chemistry: where have all the new reactions gone? J. Med. Chem. 59, 4443–4458 (2016).
Dian, L. & Marek, I. Rhodium-catalyzed arylation of cyclopropenes based on asymmetric direct functionalization of three-membered carbocycles. Angew. Chem. Int. Ed. 57, 3682–3686 (2018).
Wiberg, K. B. The concept of strain in organic chemistry. Angew. Chem. Int. Ed. 25, 312–322 (1986).
Menard, F. & Lautens, M. Chemodivergence in enantioselective desymmetrization of diazabicycles: ring-opening versus reductive arylation. Angew. Chem. Int. Ed. 47, 2085–2088 (2008).
Bexrud, J. & Lautens, M. A rhodium IBiox[(-)-menthyl] complex as a highly selective catalyst for the asymmetric hydroarylation of azabicyles: an alternative route to epibatidine. Org. Lett. 12, 3160–3163 (2010).
So, C. M., Kume, S. & Hayashi, T. Rhodium-catalyzed asymmetric hydroarylation of 3-pyrrolines giving 3-arylpyrrolidines: protonation as a key step. J. Am. Chem. Soc. 135, 10990–10993 (2013).
Celanire, S. & Poli, S. (eds) Small Molecule Therapeutics for Schizophrenia Vol. 13 (Springer, 2015).
Buter, J., Moezelaar, R. & Minnaard, A. J. Enantioselective palladium catalyzed conjugate additions of ortho-substituted arylboronic acids to β,β-disubstituted cyclic enones: total synthesis of herbertenediol, enokipodin A and enokipodin B. Org. Biomol. Chem. 12, 5883–5890 (2014).
Hayashi, T., Takahashi, M., Takaya, Y. & Ogasawara, M. Catalytic cycle of rhodium-catalyzed asymmetric 1,4-addition of organoboronic acids. Arylrhodium, oxa-π-allylrhodium, and hydroxorhodium intermediates. J. Am. Chem. Soc. 124, 5052–5058 (2002).
Sommer, H., Juliá-Hernández, F., Martin, R. & Marek, I. Walking metals for remote functionalization. ACS Cent. Sci. 4, 153–165 (2018).
Oguma, K., Miura, M., Satoh, T. & Nomura, M. Merry-go-round multiple alkylation on aromatic rings via rhodium catalysis. J. Am. Chem. Soc. 122, 10464–10465 (2000).
Miller, L. C. & Sarpong, R. Divergent reactions on racemic mixtures. Chem. Soc. Rev. 40, 4550–4562 (2011).
Steiner, G., Unger, L., Behl, B., Teschendorf, H.-J. & Munschauer, R. N-substituted 3-azabicyclo[3.2.0]heptane derivates useful as neuroleptic agents, etc. WIPO patent WO1994000458A1 (1994).
Steiner, G., Munschauer, R., Klebe, G. & Siggel, L. Diastereoselective synthesis of exo-6-aryl-3-azabicyclo[3.2.0]heptane derivatives by intramolecular [2+2] photocycloadditions of diallylic amines. Heterocycles 40, 319–330 (1995).
Denisenko, A. V. et al. Photochemical synthesis of 3-azabicyclo[3.2.0]heptanes: advanced building blocks for drug discovery. J. Org. Chem. 82, 9627–9636 (2017).
Meyers, M. J. et al. Discovery of novel spirocyclic inhibitors of fatty acid amide hydrolase (FAAH). Part 2. Discovery of 7-azaspiro[3.5]nonane urea PF-04862853, an orally efficacious inhibitor of fatty acid amide hydrolase (FAAH) for pain. Bioorg. Med. Chem. Lett. 21, 6545–6553 (2011).
Panteleev, J., Menard, F. & Lautens, M. Ligand control in enantioselective desymmetrization of bicyclic hydrazines: rhodium(i)-catalyzed ring-opening versus hydroarylation. Adv. Synth. Catal. 350, 2893–2902 (2008).
Acknowledgements
F.W.G. and S.P.F. thank M. Mortimore (Vertex Pharmaceuticals) for discussion. F.W.G. is grateful to the National Research Fund, Luxembourg, for an AFR PhD Grant (11588566), the EPSRC Doctoral Training Partnership (DTP) for a studentship (EP/N509711/1) and Vertex Pharmaceuticals for financial support. L.v.D. is grateful for a studentship from the EPSRC Centre for Doctoral Training in Synthesis for Biology and Medicine (EP/L015838/1). F.W.G. thanks N. Amin for his help with NMR spectroscopy and S. Karabiyikoglu and S. Mishra for discussion.
Author information
Authors and Affiliations
Contributions
F.W.G. conceived the project and S.P.F. guided the research. F.W.G., A.M.L.H. and L.v.D. performed the experimental work. All authors analysed the data and planned experiments. F.W.G. and S.P.F. wrote the manuscript with contributions from all other authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Chemistry thanks Ilan Marek and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
All Supplementary Information including general methods, detailed experimental and analytical data, NMR spectra and SFC chromatograms.
Rights and permissions
About this article
Cite this article
Goetzke, F.W., Hell, A.M.L., van Dijk, L. et al. A catalytic asymmetric cross-coupling approach to the synthesis of cyclobutanes. Nat. Chem. 13, 880–886 (2021). https://doi.org/10.1038/s41557-021-00725-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-021-00725-y