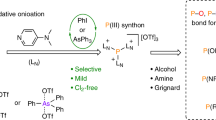
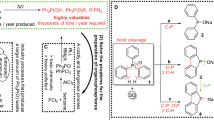
Abstract
Monophosphorus compounds are of enormous industrial importance due to the crucial roles they play in applications such as pharmaceuticals, photoinitiators and ligands for catalysis, among many others. White phosphorus (P4) is the key starting material for the preparation of all such chemicals. However, current production depends on indirect and inefficient, multi-step procedures. Here, we report a simple, effective ‘one-pot’ synthesis of a wide range of organic and inorganic monophosphorus species directly from P4. Reduction of P4 using tri-n-butyltin hydride and subsequent treatment with various electrophiles affords compounds that are of key importance for the chemical industry, and it requires only mild conditions and inexpensive, easily handled reagents. Crucially, we also demonstrate facile and efficient recycling and ultimately catalytic use of the tributyltin reagent, thereby avoiding the formation of substantial Sn-containing waste. Accessible, industrially relevant products include the fumigant PH3, the reducing agent hypophosphorous acid and the flame-retardant precursor tetrakis(hydroxymethyl)phosphonium chloride.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within the paper and its Supplementary Information files.
References
Gleason, W. An introduction to phosphorus: history, production and application. JOM 59, 17–19 (2007).
Geeson, M. B. & Cummins, C. C. Let’s make white phosphorus obsolete. ACS Cent. Sci. 6, 848–860 (2020).
Geeson, M. B. & Cummins, C. C. Phosphoric acid as a precursor to chemicals traditionally synthesized from white phosphorus. Science 359, 1383–1385 (2018).
Geeson, M. B., Ríos, P., Transue, W. J. & Cummins, C. C. Orthophosphate and sulfate utilization for C–E (E=P, S) bond formation via trichlorosilyl phosphide and sulphide anions. J. Am. Chem. Soc. 141, 6375–6384 (2019).
Scholz, R. W., Roy, A. H., Brand, F. S., Hellums, D. & Ulrich, A. E. Sustainable Phosphorus Management (Springer, 2014)
Ohtake, H. & Tsuneda, S. Phosphorus Recovery and Recycling (Springer, 2019).
Corbridge, D. E. C. Phosphorus 2000. Chemistry, Biochemistry and Technology (Elsevier, 2000).
Diskowski, H. & Hofmann, T. Phosphorus in Ullmann’s Encyclopedia of Industrial Chemistry (Wiley, 2000).
Svara, J., Weferling, N. & Hofmann, T. Phosphorus compounds, organic in Ullmann’s Encyclopedia of Industrial Chemistry (Wiley, 2006).
Bettermann, G., Krause, W., Riess, G. & Hofmann, T. Phosphorus compounds, inorganic in Ullmann’s Encyclopedia of Industrial Chemistry (Wiley, 2000).
Cossairt, B. M., Piro, N. A. & Cummins, C. C. Early-transition-metal-mediated activation and transformation of white phosphorus. Chem. Rev. 110, 4164–4177 (2010).
Caporali, M., Gonsalvi, L., Rossin, A. & Peruzzini, M. P4 activation by late-transition metal complexes. Chem. Rev. 110, 4178–4235 (2010).
Scheer, M., Balázs, G. & Seitz, A. P4 activation by main group elements and compounds. Chem. Rev. 110, 4236–4256 (2010).
Xu, L., Chi, Y., Du, S., Zhang, W.-X. & Xi, Z. Direct synthesis of phospholyl lithium from white phosphorus. Angew. Chem. Int. Ed. 55, 9187–9190 (2016).
Becker, G. et al. Tris(trimethylsilyl)phosphine and lithium bis(trimethylsilyl)phosphide bis(tetrahydrofuran). Inorg. Synth. 27, 243–349 (1990).
Bhattacharrya, K. X., Dreyfuss, S., Saffon-Merceron, N. & Mézailles, N. P4 functionalization by hydrides: direct synthesis of P–H bonds. Chem. Commun. 52, 5179–5182 (2016).
Barton, D. H. R. & Zhu, J. Elemental white phosphorus as a radical trap: a new and general route to phosphonic acids. J. Am. Chem. Soc. 115, 2071–2072 (1993).
Barton, D. H. R. & Embse, R. A. V. The invention of radical reactions. Part 39. The reaction of white phosphorus with carbon-centred radicals. An improved procedure for the synthesis of phosphonic acids and further mechanistic insights. Tetrahedron. 54, 12475–12496 (1998).
Cossairt, B. M. & Cummins, C. C. Radical synthesis of trialkyl, triaryl, trisilyl and tristannyl phosphines from P4. New J. Chem. 34, 1533–1536 (2010).
Ghosh, S. K., Cummins, C. C. & Gladysz, J. A. A direct route from white phosphorus and fluorous alkyl and aryl iodides to the corresponding trialkyl- and triarylphosphines. Org. Chem. Front. 5, 3421–3429 (2018).
Lennert, U. et al. Direct catalytic transformation of white phosphorus into arylphosphines and phosphonium salts. Nat. Catal. 2, 1101–1106 (2019).
Arockiam, P. B. et al. Versatile visible-light-driven synthesis of asymmetrical phosphines and phosphonium salts. Chem. Eur. J. 26, 16374–16382 (2020).
Lu, G. et al. Visible-light-mediated direct synthesis of phosphorotrithioates as potent anti-inflammatory agents from white phosphorus. Org. Chem. Front. 6, 190–194 (2019).
Riesel, L., Kant, M. & Helbing, R. Zur Direktsynthese von Trialk(ar)ylphosphiten und Trithiophosphiten aus elementarem Phosphor. Z. Anorg. Allg. Chem. 580, 217–223 (1990).
Mathiasch, B. & Dräger, M. Decamethyl-1λ3, 4λ3-diphospha-2,3,5,6,7-pentastannabicyclo[2.2.1]heptane, a bicyclic compound rich in tin. Angew. Chem. Int. Ed. 17, 767–768 (1978).
Mathiasch, B. Pentakis(dimethylzinn)diphosphid, struktur und kernresonanzspektren eines zinnreichen bicyclus. J. Organomet. Chem. 165, 295–301 (1979).
Dräger, M. & Mathiasch, B. Dodecamethyl-1λ3,4λ3-diphospha-2,3,5,6,7,8-hexastannabicyclo[2.2.2]octane, a highly symmetrical cage molecule. Angew. Chem. Int. Ed. 20, 1029–1030 (1981).
Brenner, A. & Riddell, G. Nickel plating on steel by chemical reduction. J. Res. Natl Bur. Stand. 37, 31–34 (1946).
Schmidbaur, H., Deschler, U., Milewski-Mahrla, B. & Zimmer-Gasser, B. Phosphonium‐benzylide und alkali‐[phosphoniumbis(benzylide)]: beispiele für salzfreie ylide und korrespondierende alkalikomplexe. Chem. Ber. 114, 608–619 (1981).
Vullo, W. J. Hydroxymethyl replacement reactions of tetrakis(hydroxymethyl)phosphonium chloride. Ind. Eng. Chem. Proc. Res. Dev. 5, 346–349 (1966).
Kuivila, H. Reduction of organic compounds by organotin hydrides. Synthesis 1970, 499–509 (1970).
Neumann, W. P. Tri-n-butyltin hydride as reagent in organic synthesis. Synthesis 1987, 665–683 (1987).
Pereyre, M., Quintard, J.-P. & Rahm, A. Tin in Organic Synthesis (Butterworths, 1987).
Rajanbabu, T. V., Bulman Page, P. C. & Buckley, B. R. Tri-n-butylstannane in Encyclopedia of Reagents for Organic Synthesis (Wiley, 2004).
Cummins, C. C. et al. The stannylphosphide anion reagent sodium bis(triphenylstannyl) phosphide: synthesis, structural characterization, and reactions with indium, tin and gold electrophiles. Inorg. Chem. 53, 3678–3687 (2014).
Norman, A. D. The synthesis of (trimethylstannyl)phosphine: the observation of phosphorus-tin nuclear spin–spin coupling. J. Organometal. Chem. 28, 81–86 (1971).
Schumann, H. Organogermyl, organostannyl, and organoplumbyl phosphines, arsines, stibines, and bismuthines. Angew. Chem. Int. Ed. 8, 937–950 (1969).
Peruzzini, M., de los Rios, I., Romerosa, A. & Vizza, F. Metal-assisted P-H bond formation: a step towards the hydrogenation of white phosphorus. Eur. J. Inorg. Chem. 2001, 593–608 (2001).
Gafurov, Z. N., Kagilev, A. A., Kantyukov, A. O., Sinyashin, O. G. & Yakharov, D. G. Hydrogenation reaction pathways in chemistry of white phosphorus. Pure Appl. Chem. 91, 797–810 (2019).
Chanon, M. & Tobe, M. L. ETC: a mechanistic concept for inorganic and organic chemistry. Angew. Chem. Int. Ed. 21, 1–23 (1982).
Chanon, K. Electron-transfer catalysis applied to organometallics. Part I. Application to the activation of Csp3-X bonds and other σ-bonded species. Bull. Soc. Chim. Fr. 1982, 197–238 (1982).
Julliard, M. & Chanon, M. Photoelectron-transfer catalysis: its connections with thermal and electrochemical analogues. Chem. Rev. 83, 425–506 (1983).
Simpkins, N. S. Azobisisobutyronitrile in Encyclopedia of Reagents for Organic Synthesis (Wiley, 2001).
Kates, S. A. & Albericio, F. 1,1′‐Azobis‐1‐cyclohexanenitrile in Encyclopedia of Reagents for Organic Synthesis (Wiley, 2001).
Montanari, F. et al. 2,2,6,6‐Tetramethylpiperidin‐1‐oxyl in Encyclopedia of Reagents for Organic Synthesis (Wiley, 2016).
Sato, A., Yorimitsu, H. & Oshima, K. Radical phosphination of organic halides and alkyl imidazole-1-carbothioates. J. Am. Chem. Soc. 128, 4240–4241 (2006).
Huber, A. et al. Phosphorus-functionalized bis(acyl)phosphane oxides for surface modification. Angew. Chem. Int. Ed. 51, 4648–4652 (2012).
Becker, G., Rössler, M. & Uhl, W. Acyl- und alkylidenphosphane. XII. Synthese und eigenschaften des 2,2-dimethylpropionylphosphans und einiger derivate. Z. Anorg. Allg. Chem. 473, 7–19 (1981).
Becker, G., Rössler, M. & Uhl, G. Acyl- und alkylidenphosphane. XX. Bis(2,2-dimethylpropionyl)phosphan und bis(2,2dimethylpropionyl)phosphide. Z. Anorg. Allg. Chem. 495, 73–88 (1982).
Becker, G. Bildung und eigenschaften von acylphosphinen. II. Verbindungen aus der reaktion von tris(trimethylsilyl)phosphin mit pivaloylchlorid. Z. Anorg. Allg. Chem. 430, 66–76 (1977).
Verlhac, J.-B. & Quintard, J.-P. N,N-dialkylaminomethyltributyltins as precursors of (N,N-dialkylaminomethyl) ketones. Tetrahedron Lett. 27, 2361–2364 (1986).
Vaillard, S. E., Mück-Lichtenfeld, C., Grimme, S. & Studer, A. Homolytic substitution at phosphorus for the synthesis of alkyl and aryl phosphanes. Angew. Chem. Int. Ed. 46, 6533–6536 (2007).
Borger, J. E., Ehlers, A. W., Slootweg, J. C. & Lammertsma, K. Functionalization of P4 through direct P–C bond formation. Chem. Eur. J. 23, 11738–11746 (2017).
Katti, K. V., Gali, H., Smith, C. J. & Berning, D. E. Design and development of functionalized water-soluble phosphines: catalytic and biomedical implications. Acc. Chem. Res. 32, 9–17 (1999).
Chen, M.-J. et al. Inherently flame-retardant flexible polyurethane foam with a low content of phosphorus-containing cross-linking agent. Ind. Eng. Chem. Res. 53, 1160–1171 (2014).
Akbayeva, D. N., Faisova, F. K. H., Abdreimova, R. R. & Peruzzini, M. Oxidation of white phosphorus by peroxides in aqueous and alcoholic solutions: mechanistic aspects and catalytic studies. J. Mol. Catal. A 267, 181–193 (2007).
Le Grognec, E., Chrétien, J.-M., Zammattio, F. & Quintard, J.-P. Methodologies limiting or avoiding contamination by organotin residues in organic synthesis. Chem. Rev. 115, 10207–10260 (2015).
Tsangaris, J. M., Willem, R. & Gielen, M. in Patai’s Chemistry of Functional Groups (ed. Saul Patai) Ch. 10 (Wiley, 2009).
Lawrence, N. J., Drew, M. D. & Bushell, S. M. Polymethylhydrosiloxane: a versatile reducing agent for organic synthesis. J. Chem. Soc. Perkin Trans. 1, 3381–3391 (1999).
Hayashi, K., Iyoda, J. & Shiihara, I. Reaction of organotin oxides, alkoxides and acyloxides with organosilicon hydrides. New preparative method of organotin hydrides. J. Organomet. Chem. 10, 81–94 (1967).
Lipowitz, J. & Bowman, S. A. Use of polymethylhydrosiloxane as a selective, neutral reducing agent for aldehydes, ketones, olefins and aromatic nitro compounds. J. Org. Chem. 38, 162–165 (1973).
Barnard, J. H., Brown, P. A., Shuford, K. L. & Martin, C. D. 1,2-Phosphaborines: hybrid inorganic/organic P–B analogues of benzene. Angew. Chem. Int. Ed. 54, 12083–12086 (2015).
Acknowledgements
We thank O. Garcia Mancheño, K. Zeitler and J. J. Weigand for valuable discussions. Funding by the European Research Council (ERC CoG 772299) and the Alexander von Humboldt Foundation (postdoctoral fellowship for D.J.S.) is gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
D.J.S. developed the hydrostannylation procedures, developed initial procedures for the formation of final products, and performed mechanistic studies. D.J.S. and J.C. optimized the synthesis, isolation and purification of products at increased scale, and the recovery and recycling of Bu3Sn-based by-products. D.J.S. and M.S. developed the catalytic synthesis of THPC. D.J.S. and R.W. conceived, oversaw and directed the project. D.J.S. prepared the manuscript. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
A patent covering all of the results described herein has been filed (as of 13 February 2020) by the University of Regensburg (EP 20,157,197.3; inventors, D.J.S. and R.W.). The authors declare no other competing interests.
Additional information
Peer review information Nature Chemistry thanks Jean-Paul Quintard, Willem Schipper and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 1H NMR spectrum for the photoreaction of P4 with 6 equiv. Bu3SnH.
The reaction was performed in PhMe and driven by 455 nm LED irradiation for 18 hours prior to acquisition, as described in the Methods section. Solvent resonances are marked with an asterisk and are truncated for clarity. The inset shows an expansion of the doublet resonance with 117/119Sn satellites attributed to the PH moiety of (Bu3Sn)2PH (3).
Extended Data Fig. 2 31P{1H} NMR spectrum for the photoreaction of P4 with 6 equiv. Bu3SnH.
The reaction was performed in PhMe and driven by 455 nm LED irradiation for 18 hours prior to acquisition, as described in the Methods section. The insets show expansions of the signals attributed to Bu3SnPH2 (2) and (Bu3Sn)2PH (3), and to (Bu3Sn)3P (4), highlighting the presence of 117/119Sn satellites.
Extended Data Fig. 3 31P NMR spectrum for the photoreaction of P4 with 6 equiv. Bu3SnH.
The reaction was performed in PhMe and driven by 455 nm LED irradiation for 18 hours. In this case, the reaction was performed in a sealed NMR tube fitted with a J. Young valve (see Supplementary Method 16), to avoid loss of PH3 (1) during manipulation. The insets show expansions of the signals attributed to PH3 (1), and to Bu3SnPH2 (2) and (Bu3Sn)2PH (3), highlighting their multiplicity due to 1J(31P-1H) couplings.
Extended Data Fig. 5 31P{1H} NMR spectra for the photoreaction of P4 with 6 equiv. Bu3SnH in various solvents.
Reactions were otherwise identical to the example given in the Methods section and were driven by 455 nm LED irradiation for 20 hours.
Extended Data Fig. 6 Proposed balanced, overall equations for the formation of [Bn4P]Br (14).
a, In the absence of KHMDS, formation of PH3 as a stoichiometric byproduct is proposed to occur. b, In the presence of KHMDS, 14 is proposed to be the only stoichiometric phosphorus-containing product.
Extended Data Fig. 7 A proposed, outline mechanism for the catalytic transformation of P4 into THPC (17) via THP (16).
Hydrostannylation of P4 by Bu3SnH is followed by insertion of formaldehyde into P–Sn and P–H bonds. Solvolysis of the resulting Sn–O bonds releases THP, which is transformed into THPC upon eventual quenching with HCl. This step also releases Bu3SnOEt which can react with PMHS to regenerate Bu3SnH and thereby close the catalytic cycle.
Supplementary information
Supplementary Information
Characterization data, Supplementary Methods 1–42, Figs. 1–139 and Table 1.
Rights and permissions
About this article
Cite this article
Scott, D.J., Cammarata, J., Schimpf, M. et al. Synthesis of monophosphines directly from white phosphorus. Nat. Chem. 13, 458–464 (2021). https://doi.org/10.1038/s41557-021-00657-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-021-00657-7