Abstract
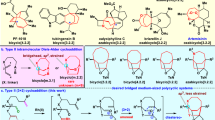
Medium-sized rings, including those embedded in bridged and fused bicyclic scaffolds, are common core structures of myriad bioactive molecules. Among various synthetic strategies towards their synthesis, intermolecular higher-order cycloaddition provides great potential to build complex medium-sized rings from simple building blocks. Unfortunately, such transformations are often plagued with competitive reaction pathways and low levels of site- and stereoselectivity. Herein, we report catalyst-controlled divergent access to three classes of medium-sized bicyclic compounds in high efficiency and stereoselectivity, by palladium-catalysed cycloadditions of tropones with γ-methylidene-δ-valerolactones. Mechanistic studies and density functional theory calculations disclosed that the divergent reactions stem from the different reaction profiles of the diastereomeric intermediates. While one undergoes either O- or C-allylation to provide [5.5.0] or [4.4.1] bicyclic compounds, the unique conformation of the other diastereomer allows an unconventional alkene isomerization to deliver bridgehead alkene-containing bicyclo[4.4.1] compounds. The conversion of these products to diverse complex polycyclic scaffolds has also been demonstrated.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data generated and analysed during this study are included in this article and its Supplementary Information files. Crystallographic data have been deposited at the Cambridge Crystallographic Data Centre (CCDC) as CCDC 1920222 (3a), 1920223 (4a), 1920224 (5a), 1920225 (5rh), 1920226 (7a), 1920227 (9a), 1920228 (11a) and 1920233 (5q) and can be obtained free of charge from the CCDC via https://www.ccdc.cam.ac.uk/.
References
Devon, T. K. Handbook of Naturally Occurring Compounds Vol. 1 (Elsevier, 2012).
Takasaki, M. et al. Inhibitors of skin-tumor promotion. VIII. Inhibitory effects of euglobals and their related compounds on Epstein–Barr virus activation. Chem. Pharm. Bull. 38, 2737–2739 (1990).
Tran, D. N. & Cramer, N. Biomimetic synthesis of (+)-ledene, (+)-viridiflorol, (−)-palustrol, (+)-spathulenol, and psiguadial A, C, and D via the platform terpene (+)-bicyclogermacrene. Chem. Eur. J. 20, 10654–10660 (2014).
Kuwajima, I. & Tanino, K. Total synthesis of ingenol. Chem. Rev. 105, 4661–4670 (2005).
Jørgensen, L. et al. 14-Step synthesis of (+)-ingenol from (+)-3-carene. Science 341, 878–882 (2013).
Amagata, T. et al. Unusual C25 steroids produced by a sponge-derived Penicillium citrinum. Org. Lett. 5, 4393–4396 (2003).
Liu, J. et al. Asymmetric total synthesis of cyclocitrinol. J. Am. Chem. Soc. 140, 5365–5369 (2018).
Anslyn, E. V. & Dougherty, D. A. Modern Physical Organic Chemistry (University Science Books, 2006).
Yet, L. Metal-mediated synthesis of medium-sized rings. Chem. Rev. 100, 2963–3008 (2000).
Rigby, J. H. Transition metal promoted higher-order cycloaddition reactions in organic synthesis. Acc. Chem. Res. 26, 579–585 (1993).
Palazzo, T. A., Mose, R. & Jørgensen, K. A. Cycloaddition reactions: why is it so challenging to move from six to ten electrons? Angew. Chem. Int. Ed. 56, 10033–10038 (2017).
Palazzo, T. A. & Jørgensen, K. A. Higher-order cycloaddition reactions: a computational perspective. Tetrahedron 74, 7381–7387 (2018).
Trost, B. M., McDougall, P. J., Hartmann, O. & Wathen, P. T. Asymmetric synthesis of bicyclo[4.3.1]decadienes and bicyclo[3.3.2]decadienes via [6+3] trimethylenemethane cycloaddition with tropones. J. Am. Chem. Soc. 130, 14960–14961 (2008).
Trost, B. M. & McDougall, P. J. Access to a welwitindolinone core using sequential cycloadditions. Org. Lett. 11, 3782–3785 (2009).
Liu, H. et al. Metal-catalyzed [6+3] cycloaddition of tropone with azomethine ylides: a practical access to piperidine-fused bicyclic heterocycles. J. Am. Chem. Soc. 136, 2625–2629 (2014).
Teng, H. –L., Yao, L. & Wang, C. –J. Cu(i)-catalyzed regio- and stereoselective [6+3] cycloaddition of azomethine ylides with tropone: an efficient asymmetric access to bridged azabicyclo[4.3.1]decadienes. J. Am. Chem. Soc. 136, 4075–4080 (2014).
Xie, M. et al. Catalytic asymmetric [8+2] cycloaddition: synthesis of cycloheptatriene-fused pyrrole derivatives. Angew. Chem. Int. Ed. 52, 5604–5607 (2013).
Wang, S., Rodríguez-Escrich, C. & Pericàs, M. A. Catalytic asymmetric [8+2] annulation reactions promoted by a recyclable immobilized isothiourea. Angew. Chem. Int. Ed. 56, 15068–15072 (2017).
Mose, R. et al. Organocatalytic stereoselective [8+2] and [6+4] cycloadditions. Nat. Chem. 9, 487–492 (2017).
Tejero, R., Ponce, A., Adrio, J. & Carretero, J. C. Ni-catalyzed [8+3] cycloaddition of tropones with 1, 1-cyclopropanediesters. Chem. Commun. 49, 10406–10408 (2013).
Zhang, J. et al. Catalytic asymmetric [8+3] annulation reactions of tropones or azaheptafulvenes with meso-aziridines. Chem. Eur. J. 24, 13428–13431 (2018).
Yu, P. et al. Organocatalytic [6+4] cycloadditions via zwitterionic intermediates: chemo-, regio-, and stereoselectivities. J. Am. Chem. Soc. 140, 13726–13735 (2018).
Inagaki, F., Sugikubo, K., Miyashita, Y. & Mukai, C. Rhodium(i)-catalyzed intramolecular [5+2] cycloaddition reactions of alkynes and allenylcyclopropanes: construction of bicyclo[5.4.0]undecatrienes and bicyclo[5.5.0]dodecatrienes. Angew. Chem. Int. Ed. 49, 2206–2210 (2010).
Mei, G., Liu, X., Qiao, C., Chen, W. & Li, C.-C. Type II intramolecular [5+2] cycloaddition: facile synthesis of highly functionalized bridged ring systems. Angew. Chem. Int. Ed. 54, 1754–1758 (2015).
Liu, X. et al. Recent synthetic studies towards natural products via [5+2] cycloaddition reactions. Org. Chem. Front. 5, 1217–1228 (2018).
Wang, M., Rong, Z. –Q. & Zhao, Y. Stereoselective synthesis of epsilon-lactones or spiro-heterocycles through NHC-catalyzed annulation: divergent reactivity by catalyst control. Chem. Commun. 50, 15309–15312 (2014).
Yang, L. –C. et al. Construction of nine-membered heterocycles through palladium-catalyzed formal [5+4] cycloaddition. Angew. Chem. Int. Ed. 56, 2927–2931 (2017).
Rong, Z. –Q. et al. Nine-membered benzofuran-fused heterocycles: enantioselective synthesis by Pd-catalysis and rearrangement via transannular bond formation. J. Am. Chem. Soc. 139, 15304–15307 (2017).
Wang, Y. –N. et al. Pd-catalyzed enantioselective [6+4] cycloaddition of vinyl oxetanes with azadienes to access ten-membered heterocycles. Angew. Chem. Int. Ed. 57, 1596–1600 (2018).
Takeshita, H., Wada, Y., Mori, A. & Hatsui, T. The cycloaddition reaction of isobenzofuran with some tropones. Chem. Lett. 2, 335–336 (1973).
Li, P. & Yamamoto, H. Lewis acid catalyzed inverse-electron-demand Diels–Alder reaction of tropones. J. Am. Chem. Soc. 131, 16628–16629 (2009).
Hammer, N. et al. Catalytic asymmetric [4+2]-cycloadditions using tropolones: developments, scope, transformations, and bioactivity. Angew. Chem. Int. Ed. 57, 13216–13220 (2018).
Shintani, R., Murakami, M. & Hayashi, T. γ-Methylidene-δ-valerolactones as a coupling partner for cycloaddition: palladium-catalyzed [4+3] cycloaddition with nitrones. J. Am. Chem. Soc. 129, 12356–12357 (2007).
Shintani, R., Park, S., Shirozu, F., Murakami, M. & Hayashi, T. Palladium-catalyzed asymmetric decarboxylative lactamization of γ-methylidene-δ-valerolactones with isocyanates: conversion of racemic lactones to enantioenriched lactams. J. Am. Chem. Soc. 130, 16174–16175 (2008).
Shintani, R., Tsuji, T., Park, S. & Hayashi, T. Mechanistic investigation of the palladium-catalyzed decarboxylative cyclization of γ-methylidene-δ-valerolactones with isocyanates: kinetic studies and origin of the site selectivity in the nucleophilic attack at a (π-allyl)palladium. J. Am. Chem. Soc. 132, 7508–7513 (2010).
Trost, B. M. & Seoane, P. R. [6+3] Cycloaddition to nine-membered ring carbocycles. J. Am. Chem. Soc. 109, 615–617 (1987).
Wu, S., Mori, A. & Takeshita, H. Formations of an [8π + 4π] cycloadduct via an electron-transfer mechanism and a meta-cycloadduct by irradiations of tropone and 9,10-dicyanoanthracene. J. Chem. Soc. Chem. Commun. 1994, 919–920 (1994).
Zhao, Y. & Truhlar, D. G. Density functionals with broad applicability in chemistry. Acc. Chem. Res. 41, 157–167 (2007).
Zhao, Y. & Truhlar, D. G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 120, 215–241 (2008).
Haibach, M. C. & Seidel, D. C–H bond functionalization through intramolecular hydride transfer. Angew. Chem. Int. Ed. 53, 5010–5036 (2014).
Wang, L. & Xiao, J. Hydrogen-atom transfer reactions. Top. Curr. Chem. 374, 17 (2016).
Kumar, P., Thakur, A., Hong, X., Houk, K. N. & Louie, J. Ni(NHC)]-catalyzed cycloaddition of diynes and tropone: apparent enone cycloaddition involving an 8π insertion. J. Am. Chem. Soc. 136, 17844–17851 (2014).
Trost, B. M. & Van Vranken, D. L. Asymmetric transition metal-catalyzed allylic alkylations. Chem. Rev. 96, 395–422 (1996).
Trost, B. M. & Schultz, J. E. Palladium-catalyzed asymmetric allylic alkylation strategies for the synthesis of acyclic tetrasubstituted stereocenters. Synthesis 51, 1–30 (2019).
Mark, J. Y. W., Pouwer, R. H. & Williams, C. M. Natural products with anti-Bredt and bridgehead double bonds. Angew. Chem. Int. Ed. 53, 13664–13688 (2014).
Acknowledgements
We are grateful for financial support from the National University of Singapore (R-143-000-A57-114) and Ministry of Education of Singapore (R-143-000-A94-112). We also thank the Programme of Introducing Talents of Discipline to Universities for support.
Author information
Authors and Affiliations
Contributions
L.-C.Y. and Y.-N.W. designed and performed the experiments. R.L. discovered the key intermediate and implemented the mechanistic studies. Y. Lan and Y. Luo conducted the DFT calculation. X.Q.N., B.Y. and Z.-Q.R. helped with substrate synthesis and data collection. Y.Z. directed the project, analysed the results and wrote the manuscript with Y.-N.W., L.-C.Y., Y. Lan and Z.S.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
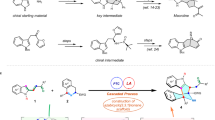
Extended Data Fig. 1 DFT calculations of all the possible cycloadducts and the transition states of C-allylation.
DFT calculations were performed using M06-2X in toluene with dppf as the ligand. The energies are shown in kcal/mol. The values of bond length are given in ångstrom. a, Calculated relative free energies of all possible cycloadducts related to their starting materials 1a and 2a. The observed cycloadducts are marked within boxes. The Gibbs free energy of 3 is higher than 4 and 5. The bridgehead olefin-containing 5a is the most thermodynamic stable cycloadduct. b, Dihedral angle of DC-C2-C1-C11 is the crucial factor to determine divergent synthesis of 4a or 5a. TS-V and TS-VII with flat dihedral angle are more favoured. c, The conformation of 4a’-1 shows that one of H at bridgehead is close to C7 which is proposed to facilitate a rapid 1,5-H shift to deliver 5a.
Extended Data Fig. 2 DFT calculation of TS-II and TS-III for selective formation of 5 vs 4 for 5m-5p.
In contrast to the model substrate, TS-III leading to 5 is favoured over TS-II leading to 4 for bulky substrates. The corresponding dihedral angles (D) in TS-III- 5 m, TS-III-5n and TS-III-5p do not change a lot from the transition state in model reaction (TS-III), while such difference of dihedral angle (DCA-C2-CB-O) from TS-II is more obvious and their energy barriers would increase a lot from TS-II as well. As an overall effect, summarized at the bottom of Fig. 4d, the energy barriers of TS-IIs for 5m-5p are 1.2-2.9 kcal/mol higher than their corresponding TS-III, in contrast to the trend of 4a/5a (–2.0 kcal/mol).
Supplementary information
Supplementary Information
General information on substrate synthesis, catalytic method procedures and mechanistic investigation, DFT calculations, complete characterization of reaction products including NMR data and spectra, high-resolution mass spectrometry, single-crystal X-ray data and high-performance liquid chromatography traces to show the enantioenrichment of the compounds, including Supplementary Figs. 1–14 and Tables 1–6.
Supplementary Table 1
B3LYP geometries for all of the cycloadducts, intermediates and transition states.
Supplementary Data 1
Crystallographic data for compound 3a (CCDC reference 1920222).
Supplementary Data 2
Crystallographic data for compound 4a (CCDC reference 1920223).
Supplementary Data 3
Crystallographic data for compound 5a (CCDC reference 1920224).
Supplementary Data 4
Crystallographic data for compound 5rh (CCDC reference 1920225).
Supplementary Data 5
Crystallographic data for compound 7a (CCDC reference 1920226).
Supplementary Data 6
Crystallographic data for compound 9a (CCDC reference 1920227).
Supplementary Data 7
Crystallographic data for compound 11a (CCDC reference 1920228).
Supplementary Data 8
Crystallographic data for compound 5q (CCDC reference 1920233).
Rights and permissions
About this article
Cite this article
Yang, LC., Wang, YN., Liu, R. et al. Stereoselective access to [5.5.0] and [4.4.1] bicyclic compounds through Pd-catalysed divergent higher-order cycloadditions. Nat. Chem. 12, 860–868 (2020). https://doi.org/10.1038/s41557-020-0503-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-020-0503-7
This article is cited by
-
Recent Advances in Palladium-Catalyzed [4 + n] Cycloaddition of Lactones, Benzoxazinanones, Allylic Carbonates, and Vinyloxetanes
Topics in Current Chemistry (2023)
-
Synthesis of cycloheptanoids through catalytic enantioselective (4 + 3)-cycloadditions of 2-aminoallyl cations with dienol silyl ethers
Nature Synthesis (2022)
-
Construction of enantioenriched eight-membered lactones via Pd-catalyzed asymmetric (6+2) dipolar annulation
Science China Chemistry (2022)
-
Direct access to spirocycles by Pd/WingPhos-catalyzed enantioselective cycloaddition of 1,3-enynes
Nature Communications (2021)