Abstract
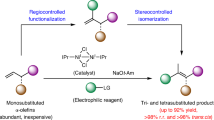
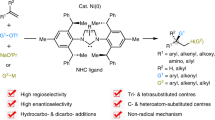
All-carbon tetrasubstituted olefins have been found in numerous biologically important compounds and organic materials. However, regio- and stereocontrolled construction of this structural motif still constitutes a significant synthetic challenge. Here, we show that a modular and regioselective synthesis of all-carbon tetrasubstituted olefins can be realized via alkenyl halide- or triflate-mediated palladium/norbornene catalysis, which is enabled by a modified norbornene containing a C2 amide moiety. This new norbornene co-catalyst effectively suppressed undesired cyclopropanation pathways, which have previously been a main obstacle for developing such reactions. Diverse cyclic and acyclic alkenyl bromides or triflates with a wide range of functional groups can be employed as substrates. Various substituents can be introduced at the alkene C1 and C2 positions regioselectively simply by changing the coupling partners. Initial mechanistic studies provide insights on the rate-limiting step as well as the structure of the actual active ligand in this system.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within the paper and its Supplementary Information. Crystallographic data for compound 4e have been deposited at the Cambridge Crystallographic Data Centre (CCDC) under deposition no. CCDC 1908383. These data can be obtained free of charge from the CCDC (http://www.ccdc.cam.ac.uk/data_request/cif).
References
Flynn, A. B. & Ogilvie, W. W. Stereocontrolled synthesis of tetrasubstituted olefins. Chem. Rev. 107, 4698–4745 (2007).
Normant, J. F. & Alexakis, A. Carbometallation (C-metallation) of alkynes – stereospecific synthesis of alkenyl derivatives. Synthesis 1981, 841–870 (1981).
Doyle, M. P. in Comprehensive Organometallic Chemistry II, Vol. 12 (eds. Abel, E. W. et al.) Ch. 5.1, 387–420 (Pergamon, 1995).
Muller, D. S. & Marek, I. Copper mediated carbometalation reactions. Chem. Soc. Rev. 45, 4552–4566 (2016).
Negishi, E., Zhang, Y., Cederbaum, F. E. & Webb, M. B. A selective method for the synthesis of stereodefined exocyclic alkenes via allylmetalation of propargyl alcohols. J. Org. Chem. 51, 4080–4082 (1986).
Itami, K., Kamei, T. & Yoshida, J. Diversity-oriented synthesis of tamoxifen-type tetrasubstituted olefins. J. Am. Chem. Soc. 125, 14670–14671 (2003).
Zhou, C. X. & Larock, R. C. Regio- and stereoselective route to tetrasubstituted olefins by the palladium-catalyzed three-component coupling of aryl iodides, internal alkynes, and arylboronic acids. J. Org. Chem. 70, 3765–3777 (2005).
Zhang, D.-H. & Ready, J. M. Iron-catalyzed carbometalation of propargylic and homopropargylic alcohols. J. Am. Chem. Soc. 128, 15050–15051 (2006).
Gericke, K. M., Chai, D. I., Bieler, N. & Lautens, M. The norbornene shuttle: multicomponent domino synthesis of tetrasubstituted helical alkenes through multiple C–H functionalization. Angew. Chem. Int. Ed. 48, 1447–1451 (2009).
Zhou, Y.-Q., You, W., Smith, K. B. & Brown, M. K. Copper-catalyzed cross-coupling of boronic esters with aryl iodides and application to the carboboration of alkynes and allenes. Angew. Chem. Int. Ed. 53, 3475–3479 (2014).
Xue, F., Zhao, J., Hor, T. S. A. & Hayashi, T. Nickel-catalyzed three-component domino reactions of aryl grignard reagents, alkynes, and aryl halides producing tetrasubstituted alkenes. J. Am. Chem. Soc. 137, 3189–3192 (2015).
Gampe, C. M. & Carreira, E. M. Arynes and cyclohexyne in natural product synthesis. Angew. Chem. Int. Ed. 51, 3766–3778 (2012).
Catellani, M., Frignani, F. & Rangoni, A. A complex catalytic cycle leading to a regioselective synthesis of o,o’‐disubstituted vinylarenes. Angew. Chem. Int. Ed. Engl. 36, 119–122 (1997).
Catellani, M., Motti, E. & Della Ca’, N. Catalytic sequential reactions involving palladacycle-directed aryl coupling steps. Acc. Chem. Res. 41, 1512–1522 (2008).
Martins, A., Mariampillai, B. & Lautens, M. Synthesis in the key of Catellani: norbornene-mediated ortho C–H functionalization. Top. Curr. Chem. 292, 1–33 (2010).
Ye, J. & Lautens, M. Palladium-catalysed norbornene-mediated C–H functionalization of arenes. Nat. Chem. 7, 863–870 (2015).
Della, Ca’,N., Fontana, M., Motti, E. & Catellani, M. Pd/Norbornene: a winning combination for selective aromatic functionalization via C–H bond activation. Acc. Chem. Res. 49, 1389–1400 (2016).
Liu, Z.-S., Gao, Q., Cheng, H.-G. & Zhou, Q. The alkylating reagents employed in catellani-type reactions. Chem. Eur. J. 24, 15461–15476 (2018).
Wang, J. & Dong, G. Palladium/Norbornene cooperative catalysis. Chem. Rev. 119, 7478–7528 (2019).
Lautens, M. & Piguel, S. A new route to fused aromatic compounds by using a palladium-catalyzed alkylation–alkenylation sequence. Angew. Chem. Int. Ed. 39, 1045–1046 (2000).
Catellani, M., Motti, E. & Baratta, S. A novel palladium-catalyzed synthesis of phenanthrenes from ortho-substituted aryl iodides and diphenyl- or alkylphenylacetylenes. Org. Lett. 3, 3611–3614 (2001).
Faccini, F., Motti, E. & Catellani, M. A new reaction sequence involving palladium-catalyzed unsymmetrical aryl coupling. J. Am. Chem. Soc. 126, 78–79 (2004).
Catellani, M. & Chiusoli, G. P. Competitive processes in palladium-catalyzed C–C bond formation. J. Organomet. Chem. 233, C21–C24 (1982).
Khanna, A., Premachandra, I. D. U. A., Sung, P. D. & Van Vranken, D. L. Palladium-catalyzed Catellani aminocyclopropanation reactions with vinyl halides. Org. Lett. 15, 3158–3161 (2013).
Blaszykowski, C., Aktoudianakis, E., Bressy, C., Alberico, D. & Lautens, M. Preparation of annulated nitrogen-containing heterocycles via a one-pot palladium-catalyzed alkylation/direct arylation sequence. Org. Lett. 8, 2043–2045 (2006).
Yamamoto, Y., Murayama, T., Jiang, J., Yasui, T. & Shibuya, M. The vinylogous Catellani reaction: a combined computational and experimental study. Chem. Sci. 9, 1191–1199 (2018).
Wang, J., Li, R., Dong, Z., Liu, P. & Dong, G. Complementary site-selectivity in arene functionalization enabled by overcoming the ortho constraint in palladium/norbornene catalysis. Nat. Chem. 10, 866–872 (2018).
Blanchot, M., Candito, D. A., Larnaud, F. & Lautens, M. Formal synthesis of nitidine and nk109 via palladium-catalyzed domino direct arylation/n-arylation of aryl triflates. Org. Lett. 13, 1486–1489 (2011).
Dong, Z., Lu, G., Wang, J., Liu, P. & Dong, G. Modular ipso/ortho difunctionalization of aryl bromides via palladium/norbornene cooperative catalysis. J. Am. Chem. Soc. 140, 8551–8562 (2018).
Zhang, H., Chen, P. & Liu, G. Palladium-catalyzed cascade C–H trifluoroethylation of aryl iodides and heck reaction: efficient synthesis of ortho-trifluoroethylstyrenes. Angew. Chem. Int. Ed. 53, 10174–10178 (2014).
Qureshi, Z., Schlundt, W. & Lautens, M. Introduction of hindered electrophiles via C–H functionalization in a palladium-catalyzed multicomponent domino reaction. Synthesis 47, 2446–2456 (2015).
Dong, Z., Wang, J., Ren, Z. & Dong, G. Ortho C–H acylation of aryl iodides by palladium/norbornene catalysis. Angew. Chem. Int. Ed. 54, 12664–12668 (2015).
Shen, P.-X., Wang, X.-C., Wang, P., Zhu, R.-Y. & Yu, J.-Q. Ligand-enabled meta-C–H alkylation and arylation using a modified norbornene. J. Am. Chem. Soc. 137, 11574–11577 (2015).
Surry, D. S. & Buchwald, S. L. Biaryl phosphane ligands in palladium-catalyzed amination. Angew. Chem. Int. Ed. 47, 6338–6361 (2008).
Wang, P. et al. Ligand-promoted meta-C–H arylation of anilines, phenols, and heterocycles. J. Am. Chem. Soc. 138, 9269–9276 (2016).
Wang, P. et al. Ligand-accelerated non-directed C–H functionalization of arenes. Nature 551, 489 (2017).
Uemura, T., Yamaguchi, M. & Chatani, N. Phenyltrimethylammonium salts as methylation reagents in the nickel-catalyzed methylation of C–H bonds. Angew. Chem. Int. Ed. 55, 3162–3165 (2016).
Catellani, M., Motti, E. & Minari, M. Symmetrical and unsymmetrical 2,6-dialkyl-1,1’-biaryls by combined catalysis of aromatic alkylation via palladacycles and suzuki-type coupling. Chem. Commun. 157–158 (2000).
Catellani, M. & Fagnola, M. C. Palladacycles as intermediates for selective dialkylation of arenes and subsequent fragmentation. Angew. Chem. Int. Ed. 33, 2421–2422 (1994).
Wilhelm, T. & Lautens, M. Palladium-catalyzed alkylation–hydride reduction sequence: synthesis of meta-substituted arenes. Org. Lett. 7, 4053–4056 (2005).
Martins, A. & Lautens, M. Aromatic ortho-benzylation reveals an unexpected reductant. Org. Lett. 10, 5095–5097 (2008).
Deledda, S., Motti, E. & Catellani, M. Palladium-catalysed synthesis of nonsymmetrically disubstituted-1,1’-biphenyls from o-substituted aryl iodides through aryl coupling and delayed hydrogenolysis. Can. J. Chem. 83, 741–747 (2005).
Catellani, M. et al. A new catalytic method for the synthesis of selectively substituted biphenyls containing an oxoalkyl chain. J. Organomet. Chem. 687, 473–482 (2003).
Liu, Z.-S. et al. Palladium/Norbornene cooperative catalysis to access tetrahydronaphthalenes and indanes with a quaternary center. ACS Catal. 8, 4783–4788 (2018).
Baba, K., Tobisu, M. & Chatani, N. Palladium-catalyzed direct synthesis of phosphole derivatives from triarylphosphines through cleavage of carbon−hydrogen and carbon−phosphorus bonds. Angew. Chem. Int. Ed. 52, 11892–11895 (2013).
Fourmy, K., Nguyen, D. H., Dechy-Cabaret, O. & Gouygou, M. Phosphole-based ligands in catalysis. Catal. Sci. Technol. 5, 4289–4323 (2015).
Trost, B. M. & Murayama, E. An approach to the phenanthrene nucleus via thionium ions and epoxyketone cyclizations. Tetrahedron Lett. 23, 1047–1050 (1982).
Acknowledgements
Financial support from the University of Chicago and NIGMS (1R01GM124414-01A1) is acknowledged. We thank K.-Y. Yoon for the X-ray crystallography.
Author information
Authors and Affiliations
Contributions
J.W., Z.D. and G.D. conceived and designed the experiments. J.W. performed experiments. C.Y. prepared a few substrates. J.W. and G.D. co-wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information
Experimental procedures, product characterization data, and mechanistic studies
Crystallographic data
Crystallographic data for compound 4e; CCDC reference 1908383
Rights and permissions
About this article
Cite this article
Wang, J., Dong, Z., Yang, C. et al. Modular and regioselective synthesis of all-carbon tetrasubstituted olefins enabled by an alkenyl Catellani reaction. Nat. Chem. 11, 1106–1112 (2019). https://doi.org/10.1038/s41557-019-0358-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-019-0358-y
This article is cited by
-
Remote-carbonyl-directed sequential Heck/isomerization/C(sp2)–H arylation of alkenes for modular synthesis of stereodefined tetrasubstituted olefins
Nature Communications (2024)
-
Stereoselective assembly of C-oligosaccharides via modular difunctionalization of glycals
Nature Communications (2024)
-
Manipulations of phenylnorbornyl palladium species for multicomponent construction of a bridged polycyclic privileged scaffold
Communications Chemistry (2022)
-
Design, synthesis, and applications of stereospecific 1,3-diene carbonyls
Science China Chemistry (2022)
-
Diversity-oriented functionalization of 2-pyridones and uracils
Nature Communications (2021)