Abstract
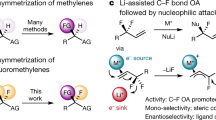
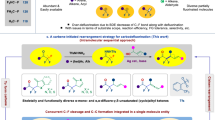
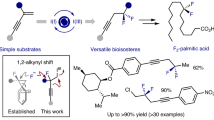
The carbon–fluorine bond engenders distinctive physicochemical properties and significant changes to general reactivity. The development of catalytic, enantioselective methods to set stereocentres that contain a benzylic C–F bond is a rapidly evolving goal in synthetic chemistry. Although there have been notable advances that enable the construction of secondary stereocentres that contain both a C–F and a C–H bond on the same carbon, significantly fewer strategies are defined to access stereocentres that incorporate a tertiary C–F bond, especially those remote from pre-existing activating groups. Here we report a general method that establishes C–F tertiary benzylic stereocentres by forging a C–C bond via a Pd-catalysed enantioselective Heck reaction of acyclic alkenyl fluorides with arylboronic acids. This method provides a platform to rapidly incorporate significant functionality about the benzylic tertiary fluoride by virtue of the diversity of both reaction partners, as well as the ability to install the stereocentres remotely from pre-existing functional groups.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
All the characterization data and experimental protocols are provided in this article and its Supplementary Information. Data are also available from the corresponding author upon request.
References
Müller, K., Faeh, C. & Diederich, F. Fluorine in pharmaceuticals: looking beyond intuition. Science 317, 1881–1886 (2007).
Purser, S., Moore, P. R., Swallow, S. & Gouverneur, V. Fluorine in medicinal chemistry. Chem. Soc. Rev. 37, 320–330 (2008).
Gillis, E. P., Eastman, K. J., Hill, M. D., Donnelly, D. J. & Meanwell, N. A. Applications of fluorine in medicinal chemistry. J. Med. Chem. 58, 8315–8359 (2015).
Zhu, Y. et al. Modern approaches for asymmetric construction of carbon−fluorine quaternary stereogenic centers: synthetic challenges and pharmaceutical needs. Chem. Rev. 118, 3887–3964 (2018).
Ma, J.-A. & Cahard, D. Asymmetric fluorination, trifluoromethylation, and perfluoroalkylation reactions. Chem. Rev. 104, 6119–6146 (2004).
Brunet, V. A. & O’Hagan, D. Catalytic asymmetric fluorination comes of age. Angew. Chem. Int. Ed. 47, 1179–1182 (2008).
Yang, X., Wu, T., Phipps, R. J. & Toste, F. D. Advances in catalytic enantioselective fluorination, mono-, di-, and trifluoromethylation, and trifluoromethylthiolation reactions. Chem. Rev. 115, 826–870 (2015).
Differding, E. & Lang, R. W. New fluorinating reagents—I. The first enantioselective fluorination reaction. Tetrahedron Lett. 29, 6087–6090 (1988).
Shibata, N., Suzuki, E., Asahi, T. & Shiro, M. Enantioselective fluorination mediated by cinchona alkaloid derivatives/selectfluor combinations: reaction scope and structural information for N-fluorocinchona alkaloids. J. Am. Chem. Soc. 123, 7001–7009 (2001).
Mohar, B., Baudoux, J., Plaquevent, J.-C. & Cahard, D. Electrophilic fluorination mediated by cinchona alkaloids: highly enantioselective synthesis of α-fluoro-α-phenylglycine derivatives. Angew. Chem. Int. Ed. 40, 4214–4216 (2001).
Marigo, M., Fielenbach, D., Braunton, A., Kjærsgaard, A. & Jørgensen, K. A. Enantioselective formation of stereogenic carbon−fluorine centers by a simple catalytic method. Angew. Chem. Int. Ed. 44, 3703–3706 (2005).
Steiner, D. D., Mase, N. & Barbas, C. F. Direct asymmetric α-fluorination of aldehydes. Angew. Chem. Int. Ed. 44, 3706–3710 (2005).
Shibatomi, K., Kitahara, K., Okimi, T., Abe, Y. & Iwasa, S. Enantioselective fluorination of α-branched aldehydes and subsequent conversion to α-hydroxyacetals via stereospecific C−F bond cleavage. Chem. Sci. 7, 1388–1392 (2016).
You, Y., Zhang, L. & Luo, S. Reagent-controlled enantioselectivity switch for the asymmetric fluorination of β-ketocarbonyls by chiral primary amine catalysis. Chem. Sci. 8, 621–626 (2017).
Shibata, N. et al. Highly enantioselective catalytic fluorination and chlorination reactions of carbonyl compounds capable of two-point binding. Angew. Chem. Int. Ed. 44, 4204–4207 (2005).
Reddy, D. S. et al. Desymmetrization-like catalytic enantioselective fluorination of malonates and its application to pharmaceutically attractive molecules. Angew. Chem. Int. Ed. 47, 164–168 (2008).
Jiao, Z. et al. Palladium-catalyzed enantioselective α-arylation of α-fluoroketones. J. Am. Chem. Soc. 138, 15980–15986 (2016).
Bélanger, É., Cantin, K., Messe, O., Tremblay, M. & Paquin, J.-F. Enantioselective Pd-catalyzed allylation reaction of fluorinated silyl enol ethers. J. Am. Chem. Soc. 129, 1034–1035 (2007).
Liang, Y. & Fu, G. C. Catalytic asymmetric synthesis of tertiary alkyl fluorides: Negishi cross-couplings of racemic α,α-dihaloketones. J. Am. Chem. Soc. 136, 5520–5524 (2014).
Han, X., Kwiatkowski, J., Xue, F., Huang, K. W. & Lu, Y. Asymmetric Mannich reaction of fluorinated ketoesters with a tryptophan-derived bifunctional thiourea catalyst. Angew. Chem. Int. Ed. 48, 7604–7607 (2009).
Xie, C., Wu, L., Han, J., Soloshonok, V. A. & Pan, Y. Assembly of fluorinated quaternary stereogenic centers through catalytic enantioselective detrifluoroacetylative aldol reactions. Angew. Chem. Int. Ed. 54, 6019–6023 (2015).
Ishimaru, T. et al. Cinchona alkaloid catalyzed enantioselective fluorination of allyl silanes, silyl enol ethers, and oxindoles. Angew. Chem. Int. Ed. 47, 4157–4161 (2008).
Wu, J. et al. A combination of directing groups and chiral anion phase-transfer catalysis for enantioselective fluorination of alkenes. Proc. Natl Acad. Sci. USA 110, 13729–13733 (2013).
Lozano, O. et al. Organocatalyzed enantioselective fluorocyclizations. Angew. Chem. Int. Ed. 50, 8105–8109 (2011).
Wolstenhulme, J. R. & Gouverneur, V. Asymmetric fluorocyclizations of alkenes. Acc. Chem. Res. 47, 3560–3570 (2014).
Rauniyar, V., Lackner, A. D., Hamilton, G. L. & Toste, F. D. Asymmetric electrophilic fluorination using an anionic chiral phase-transfer catalyst. Science 334, 1681–1684 (2011).
Shunatona, H. P., Früh, N., Wang, Y.-M., Rauniyar, V. & Toste, F. D. Enantioselective fluoroamination: 1,4-addition to conjugated dienes using anionic phase-transfer catalysis. Angew. Chem. Int. Ed. 52, 7724–7727 (2013).
Egami, H. et al. Dianionic phase-transfer catalyst for asymmetric fluoro-cyclization. J. Am. Chem. Soc. 140, 2785–2788 (2018).
Butcher, T. W. & Hartwig, J. F. Enantioselective synthesis of tertiary allylic fluorides by iridium-catalyzed allylic fluoroalkylation. Angew. Chem. Int. Ed. 57, 13125–13129 (2018).
Mei, T.-S., Patle, H. H. & Sigman, M. S. Enantioselective construction of remote quaternary stereocentres. Nature 508, 340–344 (2014).
Mei, T.-S., Werner, E. W., Burckle, A. J. & Sigman, M. S. Enantioselective redox-relay oxidative Heck arylations of acyclic alkenyl alcohols using boronic acids. J. Am. Chem. Soc. 135, 6830–6833 (2013).
Amii, H. & Uneyama, K. C–F bond activation in organic synthesis. Chem. Rev. 109, 2119–2183 (2009).
Heitz, W. & Knebelkamp, A. Synthesis of fluorostyrenes via palladium-catalyzed reactions of aromatic halides with fluoroolefins. Macromol. Chem. Rapid Commun. 12, 69–75 (1991).
Patrick, T. B., Agboka, T. Y. & Gorrell, K. Heck reaction with 3-fluoro-3-buten-2-one. J. Fluor. Chem. 129, 983–985 (2008).
Rousée, K., Bouillon, J.-P., Couve-Bonnaire, S. & Pannecoucke, X. Stereospecific synthesis of tri- and tetrasubstituted α-fluoroacrylates by Mizoroki–Heck reaction. Org. Lett. 18, 540–543 (2016).
Hirotaki, K. & Hanamoto, T. Mizoroki–Heck reaction of (1-fluorovinyl)methyldiphenylsilane with aryl iodides. J. Org. Chem. 76, 8564–8568 (2011).
Thornbury, R. T. & Toste, F. D. Palladium-catalyzed defluorinative coupling of 1-aryl-2,2-difluoroalkenes and boronic acids: stereoselective synthesis of monofluorostilbenes. Angew. Chem. Int. Ed. 55, 11629–11632 (2016).
Yang, J., Zhao, H.-W., He, J. & Zhang, C.-P. Pd-catalyzed Mizoroki–Heck reactions using fluorine-containing agents as the cross-coupling partners. Catalysts 8, 23–57 (2018).
Werner, E. W., Mei, T.-S., Burckle, A. J. & Sigman, M. S. Enantioselective Heck arylations of acyclic alkenyl alcohols using a redox-relay strategy. Science 338, 1455–1458 (2012).
Wada, S. & Jordan, R. F. Olefin insertion into a Pd–F bond: catalyst reactivation following β-F elimination in ethylene/vinyl fluoride copolymerization. Angew. Chem. Int. Ed. 56, 1820–1824 (2017).
Lee, S. H. & Schwartz, J. Stereospecific synthesis of alkenyl fluorides (with retention) via organometallic intermediates. J. Am. Chem. Soc. 108, 2445–2447 (1986).
Furuya, T. & Ritter, T. Fluorination of boronic acids mediated by silver(i) triflate. Org. Lett. 11, 2860–2863 (2009).
O’Connor, T. J. & Toste, F. D. Gold-catalyzed hydrofluorination of electron-deficient alkynes: stereoselective synthesis of β‐fluoro Michael acceptors. ACS Catal. 8, 5947–5951 (2018).
Jana, R., Pathak, T. P. & Sigman, M. S. Advances in transition metal (Pd,Ni,Fe)-catalyzed cross-coupling reactions using alkyl-organometallics as reaction partners. Chem. Rev. 111, 1417–1492 (2011).
Hilton, M. J. et al. Relative reactivity of alkenyl alcohols in the palladium-catalyzed redox-relay Heck reaction. Tetrahedron 71, 6513–6518 (2015).
Acknowledgements
The authors acknowledge financial support from the National Institutes of Health (NIGMS RO1 GM063540). J.L. thanks the Shanghai institute of Organic Chemistry, Chinese Academy of Sciences (SIOC), for a postdoctoral fellowship. Q.Y. acknowledges Shanghai Jiao Tong University for a postdoctoral fellowship.
Author information
Authors and Affiliations
Contributions
J.L. and Q.Y. performed the experiments and analysed the data. J.L, F.D.T. and M.S.S. designed the experiments. M.S.S. prepared this manuscript with feedback from F.D.T. and J.L.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information
Supplementary experimental details and compound characterization data
Rights and permissions
About this article
Cite this article
Liu, J., Yuan, Q., Toste, F.D. et al. Enantioselective construction of remote tertiary carbon–fluorine bonds. Nat. Chem. 11, 710–715 (2019). https://doi.org/10.1038/s41557-019-0289-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-019-0289-7
This article is cited by
-
Multi-site programmable functionalization of alkenes via controllable alkene isomerization
Nature Chemistry (2023)
-
Stereodefined alkenes with a fluoro-chloro terminus as a uniquely enabling compound class
Nature Chemistry (2022)
-
Stereoselective synthesis through remote functionalization
Nature Synthesis (2022)
-
Cobalt-catalysed enantioselective C(sp3)–C(sp3) coupling
Nature Catalysis (2021)
-
Palladium-catalyzed regio- and enantioselective migratory allylic C(sp3)-H functionalization
Nature Communications (2021)