Abstract
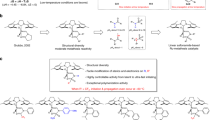
In a conventional living ring-opening metathesis polymerization (ROMP), an equal number of ruthenium complexes to the number of polymer chains synthesized are required. This can lead to high loadings of ruthenium complexes when aiming for shorter polymers. Here, a reversible chain-transfer agent was used to produce living ROMP polymers from norbornene derivatives using catalytic amounts of Grubbs’ ruthenium complexes. The polymers obtained by this method showed all of the characteristics of a living polymerization (that is, good molecular weight control, narrow molecular weight dispersities and the ability to form block copolymers). Monomers carrying functional moieties such as ferrocene, coumarin or a triisopropylsilyl-protected primary alcohol could also be catalytically polymerized in a living fashion. The method presented follows a degenerative chain-transfer process and is more economical and environmentally friendly compared with previous living ROMP procedures as it utilizes only catalytic amounts of costly and toxic ruthenium complexes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data generated or analysed during this study are included within the article and its Supplementary Information files.
References
Sutthasupa, S., Shiotsuki, M. & Sanda, F. Recent advances in ring-opening metathesis polymerization, and application to synthesis of functional materials. Polym. J. 42, 905–915 (2010).
Choi, T.-L. & Grubbs, R. H. Controlled living ring-opening-metathesis polymerization by a fast-initiating ruthenium catalyst. Angew. Chem. Int. Ed. 42, 1743–1746 (2003).
Chen, Y., Abdellatif, M. M. & Nomura, K. Olefin metathesis polymerization: some recent developments in the precise polymerizations for synthesis of advanced materials (by ROMP, ADMET). Tetrahedron 74, 619–643 (2018).
Schrock, R. R. in Handbook of Metathesis 2nd edn, Vol. 1 (eds Grubbs, R. H. & Wenzel, A. G.) 1–27 (Wiley, 2015).
Buchmeiser, M. R. Molybdenum imido, tungsten imido and tungsten oxo alkylidene N-heterocyclic carbene olefin metathesis catalysts. Chem. Eur. J. 24, 14295–14301 (2018).
Ogba, O. M., Warner, N. C., O’Leary, D. J. & Grubbs, R. H. Recent advances in ruthenium-based olefin metathesis. Chem. Soc. Rev. 47, 4510–4544 (2018).
Demel, S., Schoefberger, W., Slugovc, C. & Stelzer, F. Benchmarking of ruthenium initiators for the ROMP of a norbornenedicarboxylic acid ester. J. Mol. Catal. A 200, 11–19 (2003).
Trzaskowski, B. & Grela, K. Structural and mechanistic basis of the fast metathesis initiation by a six-coordinated ruthenium catalyst. Organometallics 32, 3625–3630 (2013).
Gu, H. et al. Tetrablock metallopolymer electrochromes. Angew. Chem. Int. Ed. 57, 2204–2208 (2018).
Liaw, D.-J., Wang, K.-L., Lee, K.-R. & Lai, J.-Y. Ring‐opening metathesis polymerization of new norbornene‐based monomers containing various chromophores. J. Polym. Sci. A 45, 3022–3031 (2007).
Riga, E. K. et al. Fluorescent ROMP monomers and copolymers for biomedical applications. Macromol. Chem. Phys. 218, 1700273 (2017).
Nguyen, H. V.-T. et al. Scalable synthesis of multivalent macromonomers for ROMP. ACS Macro Lett. 7, 472–476 (2018).
Schaefer, M., Hanik, N. & Kilbinger, A. F. M. ROMP copolymers for orthogonal click functionalizations. Macromolecules 45, 6807–6818 (2012).
Yang, S. Y. & Weck, M. Modular covalent multifunctionalization of copolymers. ACS Macro Lett. 41, 346–351 (2008).
Kumar, D. R., Lidster, B. J., Adams, R. W. & Turner, M. L. Understanding the microstructure of poly(p-phenylenevinylene)s prepared by ring-opening metathesis polymerization using 13C-labeled paracyclophanediene monomers. Macromolecules 51, 4572–4577 (2018).
Su, J. K. et al. Synthesis and mechanochemical activation of ladderene–norbornene block copolymers. J. Am. Chem. Soc. 140, 12388–12391 (2018).
Schaefer, M. et al. The role of mass and length in the sonochemistry of polymers. Macromolecules 49, 1630–1636 (2016).
Foster, J. C., Varlas, S., Blackman, L. D., Arkinstall, L. A. & O’Reilly, R. K. Ring-opening metathesis polymerization in aqueous media using a macroinitiator approach. Angew. Chem. Int. Ed. 57, 10672–10676 (2018).
Lee, H.-K. et al. Superior cascade ring-opening/ring-closing metathesis polymerization and multiple olefin metathesis polymerization: enhancing the driving force for successful polymerization of challenging monomers. J. Am. Chem. Soc. 140, 10536–10545 (2018).
Park, H. & Choi, T.-L. Fast tandem ring-opening/ring-closing metathesis polymerization from a monomer containing cyclohexene and terminal alkyne. J. Am. Chem. Soc. 134, 7270–7273 (2012).
Park, H., Lee, J.-K. & Choi, T.-L. Tandem ring-opening/ring-closing metathesis polymerization: relationship between monomer structure and reactivity. J. Am. Chem. Soc. 135, 10769–10775 (2013).
Park, H., Kang, E.-H., Müller, L. & Choi, T.-L. Versatile tandem ring-opening/ring-closing metathesis polymerization: strategies for successful polymerization of challenging monomers and their mechanistic studies. J. Am. Chem. Soc. 138, 2244–2251 (2016).
Elling, B. R. & Xia, Y. Efficient and facile end group control of living ring-opening metathesis polymers via single addition of functional cyclopropenes. ACS Macro Lett. 7, 656–661 (2018).
Pal, S., Lucarini, F., Ruggi, A. & Kilbinger, A. F. M. Functional metathesis catalyst through ring closing enyne metathesis: one pot protocol for living heterotelechelic polymers. J. Am. Chem. Soc. 140, 3181–3185 (2018).
Zhang, T., Fu, L. & Gutekunst, W. R. Practical synthesis of functional metathesis initiators using enynes. Macromolecules 41, 6497–6504 (2018).
Nagarkar, A. & Kilbinger, A. F. M. End functional ROMP polymers via degradation of a ruthenium Fischer type carbene. Chem. Sci. 5, 4687–4692 (2014).
Hilf, S., Grubbs, R. H. & Kilbinger, A. F. M. End capping ring-opening olefin metathesis polymerization polymers with vinyl lactones. J. Am. Chem. Soc. 130, 11040–11048 (2008).
Smith, D., Pentzer, E. B. & Nguyen, S. T. Bioactive and therapeutic ROMP polymers. Polym. Rev. 47, 419–459 (2007).
Sarapas, J. M., Backlund, C. M., deRonde, B. M., Minter, L. M. & Tew, G. N. ROMP- and RAFT-based guanidinium-containing polymers as scaffolds for protein mimic synthesis. Chem. Eur. J. 23, 6858–6863 (2017).
Xie, N. et al. A simple, modular synthesis of bifunctional peptide-polynorbornenes for apoptosis induction and fluorescence imaging of cancer cells. Polym. Chem. 9, 77–86 (2018).
Kang, C., Park, H., Lee, J. K. & Choi, T. L. Cascade polymerization via controlled tandem olefin metathesis/metallotropic 1,3-shift reactions for the synthesis of fully conjugated polyenynes. J. Am. Chem. Soc. 139, 11309–11312 (2017).
Kang, C., Kang, E. H. & Choi, T. L. Successful cyclopolymerization of 1,6-heptadiynes using first-generation Grubbs catalyst twenty years after its invention: revealing a comprehensive picture of cyclopolymerization using Grubbs catalysts. Macromolecules 50, 3153–3163 (2017).
Yang, S., Shin, S., Choi, I., Lee, J. & Choi, T.-L. Direct formation of large-area 2D nanosheets from fluorescent semiconducting homopolymer with orthorhombic crystalline orientation. J. Am. Chem. Soc. 139, 3082–3088 (2017).
Kovacic, S., Kren, H., Krajnc, P., Koller, S. & Slugovc, C. The use of an emulsion templated microcellular poly(dicyclopentadiene-co-norbornene) membrane as a separator in lithium-ion batteries. Macromol. Rapid Commun. 34, 581–587 (2013).
Zha, Y. P., Disabb-Miller, M. L., Johnson, Z. D., Hickner, M. A. & Tew, G. N. Metal-cation-based anion exchange membranes. J. Am. Chem. Soc. 134, 4493–4496 (2012).
Bielawski, C. W., Benitez, D., Morita, T. & Grubbs, R. H. Synthesis of end-functionalized poly(norbornene)s via ring-opening metathesis polymerization. Macromolecules 34, 8610–8618 (2001).
Liu, P., Yasir, M., Ruggi, A. & Kilbinger, A. F. M. Heterotelechelic polymers by ring-opening metathesis and regioselective chain transfer. Angew. Chem. Int. Ed. 57, 914–917 (2018).
Chiefari, J. et al. Living free-radical polymerization by reversible addition–fragmentation chain transfer: the RAFT process. Macromolecules 31, 5559–5562 (1998).
Zhang, Y. H., Keaton, R. J. & Sita, L. R. Degenerative transfer living Ziegler–Natta polymerization: application to the synthesis of monomodal stereoblock polyolefins of narrow polydispersity and tunable block length. J. Am. Chem. Soc. 125, 9062–9069 (2003).
Kempe, R. How to polymerize ethylene in a highly controlled fashion? Chem. Eur. J. 13, 2764–2773 (2007).
Valente, A., Mortreux, A., Visseaux, M. & Zinck, P. Coordinative chain transfer polymerization. Chem. Rev. 113, 3836–3857 (2013).
Ajellal, N. et al. Metal-catalyzed immortal ring-opening polymerization of lactones, lactides and cyclic carbonates. Dalton Trans. 39, 8363–8376 (2010).
Uchiyama, M., Satoh, K. & Kamigaito, M. Cationic RAFT polymerization using ppm concentrations of organic acid. Angew. Chem. Int. Ed. 54, 1924–1928 (2015).
Nagarkar, A. A. & Kilbinger, A. F. M. Catalytic living ring-opening metathesis polymerization. Nat. Chem. 7, 718–723 (2015).
Nagarkar, A. A. & Kilbinger, A. F. M. Retraction note: catalytic living ring-opening metathesis polymerization. Nat. Chem. 10, 573 (2018).
Nagarkar, A. A., Yasir, M., Crochet, A., Fromm, K. M. & Kilbinger, A. F. M. Tandem ring-opening–ring-closing metathesis for functional metathesis catalysts. Angew. Chem. Int. Ed. 55, 12343–12346 (2016).
Matson, J. B., Virgil, S. C. & Grubbs, R. H. Pulsed-addition ring-opening metathesis polymerization: catalyst-economical syntheses of homopolymers and block copolymers. J. Am. Chem. Soc. 131, 3355–3362 (2009).
Kanaoka, S. & Grubbs, R. H. Synthesis of block copolymers of silicon-containing norbornene derivatives via living ring-opening metathesis polymerization catalyzed by a ruthenium carbene complex. Macromolecules 28, 4707–4713 (1995).
Song, J.-A. & Choi, T.-L. Seven-membered ring-forming cyclopolymerization of 1,8-nonadiyne derivatives using Grubbs catalysts: rational design of monomers and insights into the mechanism for olefin metathesis polymerizations. Macromolecules 50, 2724–2735 (2017).
Doncom, K. E., Blackman, L. D., Wright, D. B., Gibson, M. I. & O’Reilly, R. K. Dispersity effects in polymer self-assemblies: a matter of hierarchical control. Chem. Soc. Rev. 46, 4119–4134 (2017).
Jenkins, A. D., Jones, R. G. & Moad, G. Terminology for reversible-deactivation radical polymerization previously called ‘controlled’ radical or ‘living’ radical polymerization (IUPAC recommendations 2010). Pure Appl. Chem. 82, 483–491 (2010).
Daeffler, C. S. & Grubbs, R. H. Catalyst-dependent routes to ring-opening metathesis alternating copolymers of substituted oxanorbornenes and cyclooctene. Macromolecules 46, 3288–3292 (2013).
Elling, B. R. & Xia, Y. Living alternating ring-opening metathesis polymerization based on single monomer additions. J. Am. Chem. Soc. 137, 9922–9926 (2015).
Acknowledgements
A.F.M.K., M.Y. and P.L. thank the Swiss National Science Foundation for funding. I.K.T. thanks the National of Competence in Research ‘Bio-inspired Nanomaterials’ for a postdoctoral fellowship.
Author information
Authors and Affiliations
Contributions
M.Y., P.L. and A.F.M.K. designed the experiments. M.Y. and P.L. performed the experiments. M.Y., I.K.T. and A.F.M.K. wrote the main manuscript text. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information
Comprehensive information on compound synthesis and characterization, 1H and 13C-NMR spectra, size exclusion chromatography traces and MALDI-ToF mass spectrometric data.
Supplementary data sets
Raw data related to the 1H and 13C-NMR spectra shown in the manuscript and the supplementary information. The NMR spectroscopic data is reported as ASCII data pairs (ppm value, intensity). Size exclusion chromatography data related to figures in the Supplementary Information is reported as ASCII data pairs (time, intensity). MALDI-ToF mass spectrometric data related to Figures in the supplementary information is reported as ASCII data pairs (m/z, intensity). Data referring to the manuscript is found in a separate folder to the supplementary information. The figure numbers are reported as part of the filename.
Rights and permissions
About this article
Cite this article
Yasir, M., Liu, P., Tennie, I.K. et al. Catalytic living ring-opening metathesis polymerization with Grubbs’ second- and third-generation catalysts. Nat. Chem. 11, 488–494 (2019). https://doi.org/10.1038/s41557-019-0239-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-019-0239-4
This article is cited by
-
Synthesis of poly(maleimide)s with promising performance via Diels–Alder reaction and ring-opening metathesis polymerization
Journal of Polymer Research (2023)
-
Tailored silyl ether monomers enable backbone-degradable polynorbornene-based linear, bottlebrush and star copolymers through ROMP
Nature Chemistry (2019)