Abstract
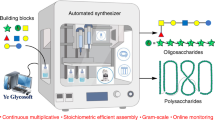
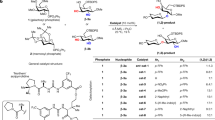
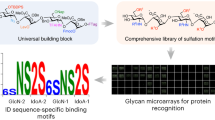
An automated platform that can synthesize a wide range of complex carbohydrates will greatly increase their accessibility and should facilitate progress in glycoscience. Here we report a fully automated process for enzyme-mediated oligosaccharide synthesis that can give easy access to different classes of complex glycans including poly-N-acetyllactosamine derivatives, human milk oligosaccharides, gangliosides and N-glycans. Our automated platform uses a catch and release approach in which glycosyltransferase-catalysed reactions are performed in solution and product purification is accomplished by solid phase extraction. We developed a sulfonate tag that can easily be installed and enables highly efficient solid phase extraction and product release using a single set of washing conditions, regardless of the complexity of the glycan. Using this custom-built synthesizer, as many as 15 reaction cycles can be performed in an automated fashion without a need for lyophilization or buffer exchange steps.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data related to this study are included in this Article and the Supplementary Information and are also available from the authors upon request.
References
Merrifield, R. B. Automated synthesis of peptides. Science 150, 178–185 (1965).
Caruthers, M. H. Gene synthesis machines: DNA chemistry and its uses. Science 230, 281–285 (1985).
Coin, I., Beyermann, M. & Bienert, M. Solid-phase peptide synthesis: from standard procedures to the synthesis of difficult sequences. Nat. Protoc. 2, 3247–3256 (2007).
Kent, S. B. Total chemical synthesis of proteins. Chem. Soc. Rev. 38, 338–351 (2009).
Plante, O. J., Palmacci, E. R. & Seeberger, P. H. Automated solid-phase synthesis of oligosaccharides. Science 291, 1523–1527 (2001).
Hahm, H. S. et al. Automated glycan assembly using the Glyconeer 2.1 synthesizer. Proc. Natl Acad. Sci. USA 114, E3385–E3389 (2017).
Tang, S. L. & Pohl, N. L. Automated solution-phase synthesis of β-1,4-mannuronate and β-1,4-mannan. Org. Lett. 17, 2642–2645 (2015).
Li, J. et al. Synthesis of many different types of organic small molecules using one automated process. Science 347, 1221–1226 (2015).
Buitrago Santanilla, A. et al. Nanomole-scale high-throughput chemistry for the synthesis of complex molecules. Science 347, 49–53 (2015).
Perera, D. et al. A platform for automated nanomole-scale reaction screening and micromole-scale synthesis in flow. Science 359, 429–434 (2018).
Gesmundo, N. J. et al. Nanoscale synthesis and affinity ranking. Nature 557, 228–232 (2018).
Piel, J. (ed.) Natural Products via Enzymatic Reactions (Springer-Verlag, Berlin, 2010).
Boltje, T. J., Buskas, T. & Boons, G. J. Opportunities and challenges in synthetic oligosaccharide and glycoconjugate research. Nat. Chem. 1, 611–622 (2009).
Unverzagt, C., Kunz, H. & Paulson, J. C. High-efficiency synthesis of sialyloligosaccharides and sialoglycopeptides. J. Am. Chem. Soc. 112, 9308–9309 (1990).
Muthana, S., Cao, H. & Chen, X. Recent progress in chemical and chemoenzymatic synthesis of carbohydrates. Curr. Opin. Chem. Biol. 13, 573–581 (2009).
Moremen, K. W. et al. Expression system for structural and functional studies of human glycosylation enzymes. Nat. Chem. Biol. 14, 156–162 (2018).
Cai, L. Recent progress in enzymatic synthesis of sugar nucleotides. J. Carbohydr. Chem. 31, 535–552 (2012).
Wang, Z. et al. A general strategy for the chemoenzymatic synthesis of asymmetrically branched N-glycans. Science 341, 379–383 (2013).
Prudden, A. R. et al. Synthesis of asymmetrical multiantennary human milk oligosaccharides. Proc. Natl Acad. Sci. USA 114, 6954–6959 (2017).
Sears, P. & Wong, C. H. Toward automated synthesis of oligosaccharides and glycoproteins. Science 291, 2344–2350 (2001).
Halcomb, R. L., Huang, H. & Wong, C.-H. Solution- and solid-phase synthesis of inhibitors of H. pylori attachment and E-selectin-mediated leukocyte adhesion. J. Am. Chem. Soc. 116, 11315–11322 (1994).
Blixt, O. & Norberg, T. Solid-phase enzymatic synthesis of a sialyl Lewis X tetrasaccharide on a sepharose matrix. J. Org. Chem. 63, 2705–2710 (1998).
Hanashima, S., Manabe, S. & Ito, Y. Divergent synthesis of sialylated glycan chains: combined use of polymer support, resin capture–release, and chemoenzymatic strategies. Angew. Chem. Int. Ed. 44, 4218–4224 (2005).
Galan, M. C., Tran, A. T. & Bernard, C. Ionic-liquid-based catch and release mass spectroscopy tags for enzyme monitoring. Chem. Commun. 46, 8968–8970 (2010).
Cai, C. et al. Fluorous-assisted chemoenzymatic synthesis of heparan sulfate oligosaccharides. Org. Lett. 16, 2240–2243 (2014).
Hwang, J. et al. Highly efficient one-pot multienzyme (OPME) synthesis of glycans with fluorous-tag assisted purification. Chem. Commun. 50, 3159–3162 (2014).
Huang, X., Witte, K. L., Bergbreiter, D. E. & Wong, C.-H. Homogenous enzymatic synthesis using a thermo-responsive water-soluble polymer support. Adv. Synth. Catal. 343, 675–681 (2001).
Matsushita, T. et al. Artificial golgi apparatus: globular protein-like dendrimer facilitates fully automated enzymatic glycan synthesis. J. Am. Chem. Soc. 132, 16651–16656 (2010).
Martin, J. G. et al. Toward an artificial golgi: redesigning the biological activities of heparan sulfate on a digital microfluidic chip. J. Am. Chem. Soc. 131, 11041–11048 (2009).
Munneke, S., Dangerfield, E. M., Stocker, B. L. & Timmer, M. S. M. The versatility of N-alkyl-methoxyamine bi-functional linkers for the preparation of glycoconjugates. Glycoconj. J. 34, 633–642 (2017).
Bode, L. Human milk oligosaccharides: every baby needs a sugar mama. Glycobiology 22, 1147–1162 (2012).
Schnaar, R. L., Gerardy-Schahn, R. & Hildebrandt, H. Sialic acids in the brain: gangliosides and polysialic acid in nervous system development, stability, disease, and regeneration. Physiol. Rev. 94, 461–518 (2014).
Liu, L., Prudden, A. R., Bosman, G. P. & Boons, G. J. Improved isolation and characterization procedure of sialylglycopeptide from egg yolk powder. Carbohydr. Res. 452, 122–128 (2017).
Joziasse, D. H. et al. Branch specificity of bovine colostrum CMP-sialic acid: Gal-β-1,4-GlcNAc-R α-2,6-sialyltransferase. Sialylation of bi-, tri-, and tetraantennary oligosaccharides and glycopeptides of the N-acetyllactosamine type. J. Biol. Chem. 262, 2025–2033 (1987).
Dube, D. H. & Bertozzi, C. R. Glycans in cancer and inflammation. Potential for therapeutics and diagnostics. Nat. Rev. Drug Discov. 4, 477–488 (2005).
Pilobello, K. T. & Mahal, L. K. Deciphering the glycocode: the complexity and analytical challenge of glycomics. Curr. Opin. Chem. Biol. 11, 300–305 (2007).
Kiessling, L. L. & Splain, R. A. Chemical approaches to glycobiology. Annu. Rev. Biochem. 79, 619–653 (2010).
Smith, D. F. & Cummings, R. D. Application of microarrays for deciphering the structure and function of the human glycome. Mol. Cell. Proteomics 12, 902–912 (2013).
Wang, L. X. & Amin, M. N. Chemical and chemoenzymatic synthesis of glycoproteins for deciphering functions. Chem. Biol. 21, 51–66 (2014).
Hsu, C. H., Hung, S. C., Wu, C. Y. & Wong, C. H. Toward automated oligosaccharide synthesis. Angew. Chem. Int. Ed. 50, 11872–11923 (2011).
Ganesh, N. V., Fujikawa, K., Tan, Y. H., Stine, K. J. & Demchenko, A. V. HPLC-assisted automated oligosaccharide synthesis. Org. Lett. 14, 3036–3039 (2012).
Crich, D. Mechanism of a chemical glycosylation reaction. Acc. Chem. Res. 43, 1144–1153 (2010).
Schmaltz, R. M., Hanson, S. R. & Wong, C. H. Enzymes in the synthesis of glycoconjugates. Chem. Rev. 111, 4259–4307 (2011).
Togayachi, A. et al. Polylactosamine on glycoproteins influences basal levels of lymphocyte and macrophage activation. Proc. Natl Acad. Sci. USA 104, 15829–15834 (2007).
Peng, W. et al. Recent H3N2 viruses have evolved specificity for extended, branched human-type receptors, conferring potential for increased avidity. Cell Host Microbe 21, 23–34 (2017).
Venkitachalam, S. et al. Biochemical and functional characterization of glycosylation-associated mutational landscapes in colon cancer. Sci. Rep. 6, 23642 (2016).
Zhang, M. et al. Association of anti-GT1a antibodies with an outbreak of Guillain–Barre syndrome and analysis of ganglioside mimicry in an associated Campylobacter jejuni strain. PLoS One 10, e0131730 (2015).
Hamasaki, H. et al. GT1b in human metastatic brain tumors: GT1b as a brain metastasis-associated ganglioside. Biochim. Biophys. Acta 1437, 93–99 (1999).
Komori, T., Imamura, A., Ando, H., Ishida, H. & Kiso, M. Study on systematizing the synthesis of the a-series ganglioside glycans GT1a, GD1a, and GM1 using the newly developed N-Troc-protected GM3 and GalN intermediates. Carbohydr. Res. 344, 1453–1463 (2009).
Ishida, H.-K., Ishida, H., Kiso, M. & Hasegawa, A. Total synthesis of ganglioside GQ1b and the related polysialogangliosides. Tetrahedron Asymm. 5, 2493–2512 (1994).
Meng, X. et al. Regioselective chemoenzymatic synthesis of ganglioside disialyl tetrasaccharide epitopes. J. Am. Chem. Soc. 136, 5205–5208 (2014).
Yu, H. et al. Sequential one-pot multienzyme chemoenzymatic synthesis of glycosphingolipid glycans. J. Org. Chem. 81, 10809–10824 (2016).
Yu, H. et al. Streamlined chemoenzymatic total synthesis of prioritized ganglioside cancer antigens. Org. Biomol. Chem. 16, 4076–4080 (2018).
Chen, Q. et al. Evidence for differential glycosylation of trophoblast cell types. Mol. Cell. Proteomics 15, 1857–1866 (2016).
Acknowledgements
This research was supported by the National Institute of General Medical Sciences (P01GM107012 and U01GM120408 to G.-J.B. and K.W.M.), the US National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The research benefitted from instrumentation provided by NIH grant S10 RR027097.
Author information
Authors and Affiliations
Contributions
T.L., L.L., K.W.M. and G.-J.B. designed the research. T.L., L.L., N.W., J.-Y.Y. and D.G.C. performed the research. J.-Y.Y. and D.G.C. contributed new reagents. T.L., L.L. and G.-J.B. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Materials and detailed Methods, Supplementary Figures 1–33, Supplementary Tables 1–21 and copies of NMR spectra.
Rights and permissions
About this article
Cite this article
Li, T., Liu, L., Wei, N. et al. An automated platform for the enzyme-mediated assembly of complex oligosaccharides. Nature Chem 11, 229–236 (2019). https://doi.org/10.1038/s41557-019-0219-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-019-0219-8
This article is cited by
-
Towards glycan foldamers and programmable assemblies
Nature Reviews Materials (2024)
-
A selective and atom-economic rearrangement of uridine by cascade biocatalysis for production of pseudouridine
Nature Communications (2023)
-
Successive remodeling of IgG glycans using a solid-phase enzymatic platform
Communications Biology (2022)
-
Automated stereocontrolled assembly-line synthesis of organic molecules
Nature Synthesis (2022)
-
Machine assembly of carbohydrates with more than 1,000 sugar units
Nature (2022)