Abstract
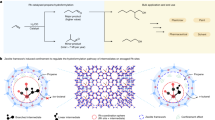
It remains difficult to understand the surface of solid acid catalysts at the molecular level, despite their importance for industrial catalytic applications. A sulfated zirconium-based metal–organic framework, MOF-808-SO4, was previously shown to be a strong solid Brønsted acid material. In this report, we probe the origin of its acidity through an array of spectroscopic, crystallographic and computational characterization techniques. The strongest Brønsted acid site is shown to consist of a specific arrangement of adsorbed water and sulfate moieties on the zirconium clusters. When a water molecule adsorbs to one zirconium atom, it participates in a hydrogen bond with a sulfate moiety that is chelated to a neighbouring zirconium atom; this motif, in turn, results in the presence of a strongly acidic proton. On dehydration, the material loses its acidity. The hydrated sulfated MOF exhibits a good catalytic performance for the dimerization of isobutene (2-methyl-1-propene), and achieves a 100% selectivity for C8 products with a good conversion efficiency.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Synthetic and experimental procedures, as well as crystallographic, spectroscopic and computational data are provided in the Supplementary Information. Crystallographic data for the structures reported in this Article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC 1871192 (MOF-808), 1871193 (MOF-808-SO4) and 1871194 (MOF-808-SeO4). Copies of the data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif. All other data supporting the findings of this study are available within the article and its Supplementary Information, or from the corresponding author upon reasonable request.
References
Arata, K. Solid superacids. Adv. Catal. 37, 165 (1990).
Ward, D. A. & Ko, E. I. One-step synthesis and characterization of zirconia–sulfate aerogels as solid superacids. J. Catal. 150, 18–33 (1994).
Haase, F. & Sauer, J. The surface structure of sulfated zirconia: periodic ab initio study of sulfuric acid adsorbed on ZrO2(101) and ZrO2(001). J. Am. Chem. Soc. 120, 13503–13512 (1998).
Bensitel, M., Saur, O., Lavalley, J. C. & Morrow, B. A. An infrared study of sulfated zirconia. Mater. Chem. Phys. 19, 147–156 (1988).
Clearfield, A., Serrette, G. P. D. & Khazi-Syed, A. H. Nature of hydrous zirconia and sulfated hydrous zirconia. Catal. Today 20, 295–312 (1994).
Kustov, L. M., Kazansky, V. B., Figueras, F. & Tichit, D. Investigation of the acidic properties of ZrO2 modified by SO4 2− anions. J. Catal. 150, 143–149 (1994).
Adeeva, V. et al. Acid sites in sulfated and metal-promoted zirconium dioxide catalysts. J. Catal. 151, 364–372 (1995).
Bolis, V., Magnacca, G., Cerrato, G. & Morterra, C. Microcalorimetric characterization of structural and chemical heterogeneity of superacid SO4/ZrO2 systems. Langmuir 13, 888–894 (1997).
Hino, M., Kurashige, M., Matsuhashi, H. & Arata, K. The surface structure of sulfated zirconia: studies of XPS and thermal analysis. Thermochim. Acta 441, 35–41 (2006).
Arata, K. Organic syntheses catalyzed by superacidic metal oxides: sulfated zirconia and related compounds. Green Chem. 11, 1719–1728 (2009).
Yadav, G. D. & Nair, J. J. Sulfated zirconia and its modified versions as promising catalysts for industrial processes. Microporous Mesoporous Mater. 33, 1–48 (1999).
Jiang, J. et al. Superacidity in sulfated metal—organic framework-808. J. Am. Chem. Soc. 136, 12844–12847 (2014).
Furukawa, H. et al. Water adsorption in porous metal–organic frameworks and related materials. J. Am. Chem. Soc. 136, 4369–4381 (2014).
Goesten, M. G. et al. Sulfation of metal–organic frameworks: opportunities for acid catalysis and proton conductivity. J. Catal. 281, 177–187 (2011).
Osborn Popp, T. M. & Yaghi, O. M. Sequence-dependent materials. Acc. Chem. Res. 50, 532–534 (2017).
Cairns, A. B. & Goodwin, A. L. Structural disorder in molecular framework materials. Chem. Soc. Rev. 42, 4881–4893 (2013).
Furukawa, H., Müller, U. & Yaghi, O. M. 'Heterogeneity within order' in metal–organic frameworks. Angew. Chem. Int. Ed. 54, 3417–3430 (2015).
Trickett, C. A. et al. Definitive molecular level characterization of defects in UiO-66 crystals. Angew. Chem. Int. Ed. 54, 11162–11167 (2015).
Åberg, M. & Glaser, J. 17O and 1H NMR study of the tetranuclear hydroxo zirconium complex in aqueous solution. Inorg. Chim. Acta 206, 53–61 (1993).
Springborg, J. Hydroxo-bridged complexes of chromium (iii), cobalt (iii), rhodium (iii), and iridium (iii). Adv. Inorg. Chem. 32, 55–169 (1988).
Hall, J. Lab Manual for Zumdahl/Zumdahl’s Chemistry 656 (Brooks Cole, Pacific Grove, 2002).
Zheng, A., Zhang, H., Lu, X., Liu, S. B. & Deng, F. Theoretical predictions of 31P NMR chemical shift threshold of trimethylphosphine oxide absorbed on solid acid catalysts. J. Phys. Chem. B 112, 4496–4505 (2008).
Zheng, A. et al. 31P chemical shift of adsorbed trialkylphosphine oxides for acidity characterization of solid acids catalysts. J. Phys. Chem. 112, 7349–7356 (2008).
Zheng, A., Huang, S. J., Liu, S. B. & Deng, F. Acid properties of solid acid catalysts characterized by solid-state 31P NMR of adsorbed phosphorous probe molecules. Phys. Chem. Chem. Phys. 13, 14889 (2011).
Chen, W. H. et al. A solid-state NMR, FT-IR and TPD study on acid properties of sulfated and metal-promoted zirconia: influence of promoter and sulfation treatment. Catal. Today 116, 111–120 (2006).
Lunsford, J. H., Sang, H., Campbell, S. M., Liang, C. H. & Anthony, R. G. An NMR study of acid sites on sulfated-zirconia catalysts using trimethylphosphine as a probe. Catal. Lett. 27, 305–314 (1994).
Gottwald, J., Demco, D. E., Graf, R. & Spiess, H. W. High-resolution double-quantum NMR spectroscopy of homonuclear spin pairs and proton connectivities in solids. Chem. Phys. Lett. 243, 314–323 (1995).
Schnell, I., Brown, S. P., Low, H. Y., Ishida, H. & Spiess, H. W. An investigation of hydrogen bonding in benzoxazine dimers by fast magic-angle spinning and double-quantum 1H NMR spectroscopy. J. Am. Chem. Soc. 120, 11784–11795 (1998).
Mahdi, H. I. & Muraza, O. Conversion of isobutylene to octane-booster compounds after methyl tert-butyl ether phaseout: the role of heterogeneous catalysis. Ind. Eng. Chem. Res. 55, 11193–11210 (2016).
Takahashi, K., Yamashita, M. & Nozaki, K. Tandem hydroformylation/hydrogenation of alkenes to normal alcohols using Rh/Ru dual catalyst or Ru single component catalyst. J. Am. Chem. Soc. 134, 18746–18757 (2012).
Behr, A. Ullman’s Encyclopedia of Industrial Chemistry 223–269 (Wiley, Hoboken, 2010).
Izquierdo, J. F., Vila, M., Tejero, J., Cunill, F. & Iborra, M. Kinetic study of isobutene dimerization catalyzed by a macroporous sulphonic acid resin. Appl. Catal. A 106, 155–165 (1993).
Kamath, R. S., Qi, Z., Sundmacher, K., Aghalayam, P. & Mahajani, S. M. Process analysis for dimerization of isobutene by reactive distillation. Ind. Eng. Chem. Res. 45, 1575–1582 (2006).
Song, X. & Sayari, A. Sulfated zirconia-based strong solid-acid catalysts: recent progress. Cat. Rev. 38, 329–412 (1996).
Himmel, D., Goll, S. K., Leito, I. & Krossing, I. A unified pH scale for all phases. Angew. Chem. Int. Ed. 49, 6885–6888 (2010).
Viggiano, A. A., Henchman, M. J., Dale, F., Deakyne, C. A. & Paulson, J. F. Gas-phase reactions of weak Brønsted bases I–, PO3 –, HSO4 –, FSO3 –, and CF3SO3 – with strong Brønsted acids H2SO4, FSO3H, and CF3SO3H, a quantitative intrinsic superacidity scale for the sulfonic acids XSO3H (X = HO, F, and CF3). J. Am. Chem. Soc. 114, 4299–4306 (1992).
Acknowledgements
This work, including the synthesis, characterization and crystal structure analysis, was funded by BASF SE and the US Department of Defense, Defense Threat Reduction Agency (HDTRA 1-12-1-0053). Work performed at the Advanced Light Source is supported by the Director, Office of Science, Office of Basic Energy Sciences, of the US Department of Energy under Contract no. DE-AC02-05CH11231. A portion of this research used resources at the Spallation Neutron Source, a DOE Office of Science User Facility operated by the Oak Ridge National Laboratory. NMR work was supported as part of the Center for Gas Separations Relevant to Clean Energy Technologies, an Energy Frontier Research Center funded by the US Department of Energy, Office of Science, Basic Energy Sciences under Award no. DE-SC0001015. T.M.O.P. acknowledges funding from the NSF Graduate Research Fellowship Program. C.Y. acknowledges support from a Hewlett-Packard Stanford Graduate Fellowship. P.U. acknowledges the German Research Foundation (DFG, PU 286/1-1). M.J.K. is grateful for financial support through the German Research Foundation (DFG, KA 4484/1-1). We acknowledge B. Rungtaweevoranit for his assistance with electron microscopy, and S. Teat and L. McCormick for the synchrotron X-ray diffraction data acquisition support at beamline 11.3.1 of the Advanced Light Source, Lawrence Berkeley National Laboratory.
Author information
Authors and Affiliations
Contributions
C.A.T. and T.M.O.P. co-wrote the manuscript. C.A.T. performed the PND modelling, single-crystal X-ray diffraction and PXRD experiments. T.M.O.P. performed the solid-state NMR experiments and NMR DFT calculations, with support and advice from J.A.R. J.S., Q.L. and J.B. performed the dimerization catalysis experiments with the support and advice of G.S. C.Y. performed the infrared experiments. J.W. performed the DFT calculations on the cluster models, with support and advice from M.P.H.-G. A.H. performed the PND experiments. P.U. performed the PXRD Rietveld refinements. M.J.K. helped with the thermogravimetric analysis experiments. J.J. supported and advised the synthesis. O.M.Y. supervised the project. All the authors reviewed and edited the manuscript and contributed to useful discussions.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Synthetic and experimental procedures; Crystallographic, spectroscopic and computational data; Supplementary Figures 1–30; Supplementary Tables 1–7; Supplementary References 1–14
Crystallographic data
CIF for MOF-808; CCDC reference: 1871192
Crystallographic data
Structure-factor file for MOF-808; CCDC: reference 1871192
Crystallographic data
CIF for MOF-808-SO4; CCDC reference: 1871193
Crystallographic data
Structure-factor file for MOF-808-SO4; CCDC reference: 1871193
Crystallographic data
CIF for MOF-808-SeO4; CCDC reference: 1871194
Crystallographic data
Structure-factor file for MOF-808-SeO4; CCDC reference: 1871194
Rights and permissions
About this article
Cite this article
Trickett, C.A., Osborn Popp, T.M., Su, J. et al. Identification of the strong Brønsted acid site in a metal–organic framework solid acid catalyst. Nature Chem 11, 170–176 (2019). https://doi.org/10.1038/s41557-018-0171-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-018-0171-z
This article is cited by
-
Recent progress on the synthesis of defective UiO-66 for thermal catalysis
Nano Research (2024)
-
From non-carbon host toward carbon-free lithium-sulfur batteries
Nano Research (2024)
-
Dynamic-static coupled sensing of trace biomarkers by molecularly imprinted metal-organic frameworks
Science China Chemistry (2023)
-
Metal–Organic Frameworks: Challenges Addressed via Magnetic Resonance Spectroscopy
Applied Magnetic Resonance (2023)
-
Activity of Brønsted Acid Sites in UiO-66 for Cyclohexanol Dehydration
Topics in Catalysis (2023)