Abstract
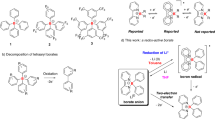
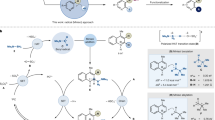
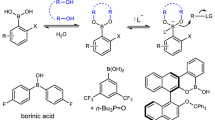
Tetracoordinate MIDA (N-methyliminodiacetic acid) boronates have found broad utility in chemical synthesis. Here, we describe mechanistic insights into the migratory aptitude of the MIDA boryl group in boron transfer processes, and show that the hemilability of the nitrogen atom on the MIDA ligand enables boron to mechanistically resemble either a hydride or a proton. The first case involves a 1,2-boryl shift, in which boron migrates as a nucleophile in its tetracoordinate form. The second case involves a neighbouring atom-promoted 1,4-boryl shift, in which boron migrates as an electrophile in its pseudo-tricoordinate form. Density functional theory studies and in situ NMR measurements all suggest that MIDA can act as a dynamic switch. These findings encouraged the development of novel migration processes involving boron that exploit the chameleonic behaviour of boron by acting as both a nucleophile and an electrophile, including the first report of a compound with a boronate functionality bound to carbon in the carboxylic acid oxidation state.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Change history
10 September 2018
During the revision of this Article prior to publication, a computational study was reported (Vallejos, M. M. & Pellegrinet, S. C. Theoretical study of the BF3-promoted rearrangement of oxiranyl N-methyliminodiacetic acid boronates. J. Org. Chem. 82, 5917–5925; 2017) that evaluates the nucleophilic boryl transfer mechanism predicted in this Article; this reference has now been added as number 19, and the subsequent references renumbered.
References
Fernandez, E. & Whiting, A. (eds) Synthesis and Application of Organoboron Compounds (Springer International, Cham, 2015).
Hall, D. G. (ed.) Boronic Acids: Preparation and Applications in Organic Synthesis, Medicine and Materials 2nd rev. edn (Wiley, Weinheim, 2011).
Diaz, D. B. & Yudin, A. K. The versatility of boron in biological target engagement. Nat. Chem. 9, 731–742 (2017).
Lennox, A. J. J. & Lloyd-Jones, G. C. Selection of boron reagents for Suzuki–Miyaura coupling. Chem. Soc. Rev. 43, 412–443 (2014).
Molander, G. A. & Ellis, N. Organotrifluoroborates: protected boronic acids that expand the versatility of the Suzuki coupling reaction. Acc. Chem. Res. 40, 275–286 (2007).
Darses, S. & Genet, J.-P. Potassium organotrifluoroborates: new perspectives in organic synthesis. Chem. Rev. 108, 288–325 (2008).
Eros, G., Kushida, Y. & Bode, J. W. A reagent for the one-step preparation of potassium acyltrifluoroborates (KATs) from aryl- and heteroarylhalides. Angew. Chem. Int. Ed. 53, 7604–7607 (2014).
Gillis, E. P. & Burke, M. D. A simple and modular strategy for small molecule synthesis: iterative Suzuki–Miyaura coupling of B-protected haloboronic acid building blocks. J. Am. Chem. Soc. 129, 6716–6717 (2007).
Gillis, E. P. & Burke, M. D. Multistep synthesis of complex boronic acids from simple MIDA boronates. J. Am. Chem. Soc. 130, 14084–14085 (2008).
He, Z. & Yudin, A. K. Amphoteric α-boryl aldehydes. J. Am. Chem. Soc. 133, 13770–13773 (2011).
He, Z., Zajdlik, A., St. Denis, J. D., Assem, N. & Yudin, A. K. Boroalkyl group migration provides a versatile entry into α-aminoboronic acid derivatives. J. Am. Chem. Soc. 134, 9926–9929 (2012).
He, Z., Trinchera, P., Adachi, S., St Denis, J. D. & Yudin, A. K. Oxidative geminal functionalization of organoboron compounds. Angew. Chem. Int. Ed. 51, 11092–11096 (2012).
Zajdlik, A. et al. α-Boryl isocyanides enable facile preparation of bioactive boropeptides. Angew. Chem. Int. Ed. 52, 8411–8415 (2013).
He, Z., Zajdlik, A. & Yudin, A. K. Air- and moisture-stable amphoteric molecules: enabling reagents in synthesis. Acc. Chem. Res. 47, 1029–1040 (2014).
St Denis, J. D., He, Z. & Yudin, A. K. Amphoteric α-boryl aldehyde linchpins in the synthesis of heterocyles. ACS Catal. 5, 5373–5379 (2015).
Adachi, S. et al. Condensation-driven assembly of boron-containing bis(heteroaryl) motifs using a linchpin approach. Org. Lett. 17, 5594–5597 (2015).
Diaz, D. B. et al. Synthesis of aminoboronic acid derivatives from amines and amphoteric boryl carbonyl compounds. Angew. Chem. Int. Ed. 55, 12659–12663 (2016).
Lee, C. F. et al. Oxalyl boronates enable modular synthesis of bioactive imidazoles. Angew. Chem. Int. Ed. 56, 6264–6267 (2017).
Vallejos, M. M. & Pellegrinet, S. C. Theoretical study of the BF3-promoted rearrangement of oxiranyl N-methyliminodiacetic acid boronates. J. Org. Chem. 82, 5917–5925 (2017).
Lee, S. J., Gray, K. C., Paek, J. S. & Burke, M. D. Simple, efficient, and modular synthesis of polyene natural products via iterative cross-coupling. J. Am. Chem. Soc. 130, 466–468 (2008).
Wang, C. & Glorius, F. Controlled iterative cross-coupling: on the way to the automation of organic synthesis. Angew. Chem. Int. Ed. 48, 2–7 (2009).
Woerly, E. M., Roy, J. & Burke, M. D. Synthesis of most polyene natural product motifs using just 12 building blocks and one coupling reaction. Nat. Chem. 6, 484–491 (2014).
Gillis, E. P. & Burke, M. D. Iterative cross-coupling with MIDA boronates: towards a general platform for small molecule synthesis. Aldrichimica Acta 42, 17–27 (2009).
St. Denis, J. D. et al. Boron-containing enamine and enamide linchpins in the synthesis of nitrogen heterocycles. J. Am. Chem. Soc. 136, 17669–17673 (2014).
Li, J. et al. Synthesis of many different types of organic small molecules using one automated process. Science 347, 1221–1226 (2015).
Gonzalez, J. A. et al. MIDA boronates are hydrolysed fast and slow by two different mechanisms. Nat. Chem. 8, 1067–1075 (2016).
Mkhalid, I. A. I., Barnard, J. H., Marder, T. B., Murphy, J. M. & Hartwig, J. F. C–H activation for the construction of C–B bonds. Chem. Rev. 110, 890–931 (2010).
Blackett, B. N., Coxon, J. M., Hartshorn, M. P. & Richards, K. E. The deuterium isotope effect for the boron trifluoride catalyzed rearrangement of 2-methyl-1,2-epoxypropane. Aust. J. Chem. 23, 839–840 (1970).
Fraile, J. M., Mayoral, J. A. & Salvatella, L. Theoretical study on the BF3-catalyzed Meinwald rearrangement reaction. J. Org. Chem. 79, 5993–5999 (2014).
Tomasi, J., Mennucci, B. & Cance, E. The IEF version of the PCM solvation method: an overview of a new method addressed to study molecular solutes at the QM ab initio level. J. Mol. Struct. 464, 211–226 (1999).
Frisch, M. J. et al. Gaussian 09, Revision C.01 (Gaussian, Inc., 2009).
Dean, J. A. (ed.) Lange’s Handbook of Chemistry 15th edn (McGraw-Hill. New York, NY, 1998).
Eberlin, L., Bertrand, C. & Whiting, A. Regioisomeric and substituent effects upon the outcome of the reaction of 1-borodienes with nitrosoarene compounds. J. Org. Chem. 80, 6574–6583 (2015).
Kisu, H., Sakaino, H., Ito, F., Yamashita, M. & Nozaki, K. A qualitative analysis of a ‘Bora-Brook rearrangement’: the ambident reactivity of boryl-substituted alkoxide including the carbon-to-oxygen migration of a boryl group. J. Am. Chem. Soc. 138, 3548–3552 (2016).
Brook, A. G. & Yu, Z. Reactions of amines with silenes and acylsilanes. Organometallics 19, 1859–1863 (2000).
Brook, A. G., MacRae, D. M. & Bassindale, A. R. The mechanism of the β-ketosilane to siloxyalkene thermal rearrangement. J. Organomet. Chem. 86, 185–192 (1975).
Blackmond, D. G. Reaction progress kinetic analysis: a powerful methodology for mechanistic studies of complex catalytic reactions. Angew. Chem. Int. Ed. 44, 4302–4320 (2005).
Blackmond, D. G. Kinetic profiling of catalytic organic reactions as a mechanistic tool. J. Am. Chem. Soc. 137, 10852–10866 (2015).
Garrett, G. E. & Taylor, M. S. A nonlinear ordinary differential equation for generating graphical rate equations from concentration versus time data. Top. Catal. 60, 554–563 (2017).
Hansch, C. & Leo, A. Substituent Constants for Correlation Analysis in Chemistry and Biology (Wiley, New York, NY, 1979).
Hansch, C., Leo, A. & Taft, R. W. A survey of Hammett substituent constants and resonance and field parameters. Chem. Rev. 91, 165–195 (1991).
Swain, C. G. & Lupton, E. C. Jr Field and resonance components of substituent effects. J. Am. Chem. Soc. 90, 4328–4337 (1968).
Swain, C. G., Unger, S. H., Rosenquist, N. R. & Swain, M. S. Substituent effects on chemical reactivity. Improved evaluation of field and resonance components. J. Am. Chem. Soc. 105, 492–502 (1983).
Brook, A. G. Molecular rearrangements of organosilicon compounds. Acc. Chem. Res. 7, 77–84 (1974).
Glendening, E. D., Reed, A. E., Carpenter, J. E. & Weinhold, F. NBO Version 3.1 (TCI, Univ. Wisconsin, Madison, WI, 1998).
Carroll, F. A. (ed.) Perspectives on Structure and Mechanism in Organic Chemistry (Brooks/Cole Publishing Company, Pacific Grove, CA, 1998).
Kende, A. S. (ed.) Organic Reactions Vol. 35 (Wiley, New York, NY, 1988).
Acknowledgements
A.K.Y. acknowledges financial support from the Natural Science and Engineering Research Council (NSERC). T.D. acknowledges the computing infrastructure provided by SHARCNET (www.sharcnet.ca). The authors also acknowledge NSERC and the Canadian Foundation for Innovation, Project Number 19119, and the Ontario Research Fund for funding of the Centre for Spectroscopic Investigation of Complex Organic Molecules and Polymers. Helpful discussions with A.P. Dicks (University of Toronto), H. Soor (University of Toronto) and C. Apte (University of Toronto) are appreciated. The authors thank D. Burns, J. Sheng and S. Nokhrin for assistance with NMR spectroscopic experiments, and H. Foy (Brock University) for assistance in the computational calculations on the 1,4-migration. C.F.L., D.B.D. and A.H. thank NSERC for PGS-D funding. S.J.K. thanks NSERC for CGS-D funding. This paper is in memory of Dr Zhi He.
Author information
Authors and Affiliations
Contributions
A.K.Y. conceived the idea. Experimental work was conducted by C.F.L., D.B.D., A.H., S.J.K. and S.K.L. Kinetic data were processed and analysed by G.E.G. Computational work was conducted by T.D. The manuscript was written by C.F.L., D.B.D. and A.K.Y.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information
Full experimental procedures, computational details and experimental data
Supplementary dataset
Eyring data processing and fitting
Supplementary dataset
Hammett data processing and fitting
Supplementary dataset
Product inhibition data processing and fitting
Calculations archive file
Raw coordinate files for the computational studies
Rights and permissions
About this article
Cite this article
Lee, C.F., Diaz, D.B., Holownia, A. et al. Amine hemilability enables boron to mechanistically resemble either hydride or proton. Nature Chem 10, 1062–1070 (2018). https://doi.org/10.1038/s41557-018-0097-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-018-0097-5
This article is cited by
-
A boryl-migratory semipinacol rearrangement
Science China Chemistry (2022)
-
Merging Boron with Nitrogen–Oxygen Bonds: A Review on BON Heterocycles
Topics in Current Chemistry (2021)
-
Regio- and stereoselective synthesis of tetra- and triarylethenes by N-methylimidodiacetyl boron-directed palladium-catalysed three-component coupling
Communications Chemistry (2019)