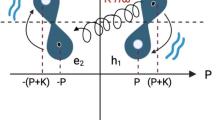
Abstract
Aromatic molecules represent fundamental building blocks in prebiotic chemistry and are contemplated as vital precursors to DNA and RNA nitrogen bases. However, despite the identification of some 300 molecules in extraterrestrial environments, the pathways to pyridine (C5H5N), pyridinyl (C5H4N·) and (iso)quinoline (C9H7N)—the simplest representative mono- and bicyclic aromatic molecules carrying nitrogen—are elusive. Here we afford compelling evidence on the gas-phase formation of methylene amidogen (H2CN·) and cyanomethyl (H2CCN·) radicals via molecular beam studies and electronic structure calculations. The modelling of the chemistries of the Taurus molecular cloud (TMC-1) and Titan’s atmosphere contemplates a complex chain of reactions synthesizing pyridine, pyridinyl and (iso)quinoline from H2CN· and H2CCN· at levels of up to 75%. This study affords unique entry points to precursors of DNA and RNA nitrogen bases in hydrocarbon-rich extraterrestrial environments thus changing the way we think about the origin of prebiotic molecules in our Galaxy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data generated in this study are available in the main text and Supplementary Information.
References
Pizzarello, S., Huang, Y. & Fuller, M. The carbon isotopic distribution of Murchison amino acids. Geochim. Cosmochim. Acta 68, 4963–4969 (2004).
Smith, K. E., Callahan, M. P., Gerakines, P. A., Dworkin, J. P. & House, C. H. Investigation of pyridine carboxylic acids in CM2 carbonaceous chondrites: potential precursor molecules for ancient coenzymes. Geochim. Cosmochim. Acta 136, 1–12 (2014).
Martins, Z. et al. Extraterrestrial nucleobases in the Murchison meteorite. Earth Planet. Sci. Lett. 270, 130–136 (2008).
Burton, A. S., Stern, J. C., Elsila, J. E., Glavin, D. P. & Dworkin, J. P. Understanding prebiotic chemistry through the analysis of extraterrestrial amino acids and nucleobases in meteorites. Chem. Soc. Rev. 41, 5459–5472 (2012).
Andrews, H., Candian, A. & Tielens, A. G. G. M. Hydrogenation and dehydrogenation of interstellar PAHs: spectral characteristics and H2 formation. Astron. Astrophys. 595, A23 (2016).
Tsuge, M., Bahou, M., Wu, Y.-J., Allamandola, L. & Lee, Y.-P. The infrared spectrum of protonated ovalene in solid para-hydrogen and its possible contribution to interstellar unidentified infrared emission. Astrophys. J. 825, 96 (2016).
Tielens, A. G. G. M. Interstellar polycyclic aromatic hydrocarbon molecules. Annu. Rev. Astron. Astrophys. 46, 289–337 (2008).
Knorke, H., Langer, J., Oomens, J. & Dopfer, O. Infrared spectra of isolated protonated polycyclic aromatic hydrocarbon molecules. Astrophys. J. 706, L66 (2009).
Hudgins, D. M., Bauschlicher, C. W. Jr & Allamandola, L. J. Variations in the peak position of the 6.2 μm interstellar emission feature: a tracer of N in the interstellar polycyclic aromatic hydrocarbon population. Astrophys. J. 632, 316 (2005).
Herbst, E. & Van Dishoeck, E. F. Complex organic interstellar molecules. Annu. Rev. Astron. Astrophys. 47, 427–480 (2009).
Kaiser, R. I. & Hansen, N. An aromatic universe—a physical chemistry perspective. J. Phys. Chem. A 125, 3826–3840 (2021).
Peeters, Z., Botta, O., Charnley, S. B., Ruiterkamp, R. & Ehrenfreund, P. The astrobiology of nucleobases. Astrophys. J. 593, L129 (2003).
Hörst, S. M. Titan’s atmosphere and climate. J. Geophys. Res. 122, 432–482 (2017).
Vuitton, V., Yelle, R. V., Klippenstein, S. J., Hörst, S. M. & Lavvas, P. Simulating the density of organic species in the atmosphere of Titan with a coupled ion–neutral photochemical model. Icarus 324, 120–197 (2019).
Loison, J. C. et al. The neutral photochemistry of nitriles, amines and imines in the atmosphere of Titan. Icarus 247, 218–247 (2015).
Kislov, V. V., Nguyen, T. L., Mebel, A. M., Lin, S. H. & Smith, S. C. Photodissociation of benzene under collision-free conditions: an ab initio/Rice–Ramsperger–Kassel–Marcus study. J. Chem. Phys. 120, 7008–7017 (2004).
Lin, M.-F. et al. Photodissociation dynamics of pyridine. J. Chem. Phys. 123, 054309 (2005).
Vuitton, V., Yelle, R. V. & McEwan, M. J. Ion chemistry and N-containing molecules in Titan’s upper atmosphere. Icarus 191, 722–742 (2007).
Ricca, A., Bauschlicher, C. W. Jr & Bakes, E. A computational study of the mechanisms for the incorporation of a nitrogen atom into polycyclic aromatic hydrocarbons in the Titan haze. Icarus 154, 516–521 (2001).
Soorkia, S. et al. Direct detection of pyridine formation by the reaction of CH (CD) with pyrrole: a ring expansion reaction. Phys. Chem. Chem. Phys. 12, 8750–8758 (2010).
López-Puertas, M. et al. Large abundances of polycyclic aromatic hydrocarbons in Titan’s upper atmosphere. Astrophys. J. 770, 132 (2013).
Anderson, C. M. & Samuelson, R. E. Titan’s aerosol and stratospheric ice opacities between 18 and 500 μm: vertical and spectral characteristics from Cassini CIRS. Icarus 212, 762–778 (2011).
Sebree, J. A., Trainer, M. G., Loeffler, M. J. & Anderson, C. M. Titan aerosol analog absorption features produced from aromatics in the far infrared. Icarus 236, 146–152 (2014).
Ali, A., Sittler, E. C. Jr, Chornay, D., Rowe, B. R. & Puzzarini, C. Organic chemistry in Titan׳ s upper atmosphere and its astrobiological consequences: I. Views towards Cassini Plasma Spectrometer (CAPS) and Ion Neutral Mass Spectrometer (INMS) experiments in space. Planet. Space Sci. 109, 46–63 (2015).
Mathé, C., Gautier, T., Trainer, M. G. & Carrasco, N. Detection opportunity for aromatic signature in Titan’s aerosols in the 4.1–5.3 μm range. Astrophys. J. Lett. 861, L25 (2018).
Zhao, L. et al. Gas-phase synthesis of corannulene—a molecular building block of fullerenes. Phys. Chem. Chem. Phys. 23, 5740–5749 (2021).
Kaiser, R. I. et al. Gas-phase synthesis of racemic helicenes and their potential role in the enantiomeric enrichment of sugars and amino acids in meteorites. Phys. Chem. Chem. Phys. 24, 25077–25087 (2022).
Parker, D. S. N. & Kaiser, R. I. On the formation of nitrogen-substituted polycyclic aromatic hydrocarbons (NPAHs) in circumstellar and interstellar environments. Chem. Soc. Rev. 46, 452–463 (2017).
Zhao, L. et al. A molecular beam and computational study on the barrierless gas phase formation of (iso)quinoline in low temperature extraterrestrial environments. Phys. Chem. Chem. Phys. 23, 18495–18505 (2021).
Broadfoot, A. L. et al. Ultraviolet spectrometer observations of Neptune and Triton. Science 246, 1459–1466 (1989).
Moores, J. E., Smith, C. L., Toigo, A. D. & Guzewich, S. D. Penitentes as the origin of the bladed terrain of Tartarus Dorsa on Pluto. Nature 541, 188–190 (2017).
Yang, Z. et al. Gas-phase formation of 1, 3, 5, 7-cyclooctatetraene (C8H8) through ring expansion via the aromatic 1, 3, 5-cyclooctatrien-7-yl radical (C8H9•) transient. J. Am. Chem. Soc. 144, 22470–22478 (2022).
Yang, Z. et al. Gas-phase formation of the resonantly stabilized 1-indenyl (C9H7•) radical in the interstellar medium. Sci. Adv. 9, eadi5060 (2023).
Zhang, J. & Valeev, E. F. Prediction of reaction barriers and thermochemical properties with explicitly correlated coupled-cluster methods: a basis set assessment. J. Chem. Theory Comput. 8, 3175–3186 (2012).
Adler, T. B., Knizia, G. & Werner, H.-J. A simple and efficient CCSD(T)-F12 approximation. J. Chem. Phys. 127, 221106 (2007).
Bourgalais, J. et al. The C(3P) + NH3 reaction in interstellar chemistry. I. Investigation of the product formation channels. Astrophys. J. 812, 106 (2015).
Chiba, S., Honda, T., Kondo, M. & Takayanagi, T. Direct dynamics study of the N(4S)+ CH3(2A2″) reaction. Comput. Theor. Chem. 1061, 46–51 (2015).
Mebel, A. M., Georgievskii, Y., Jasper, A. W. & Klippenstein, S. J. Pressure-dependent rate constants for PAH growth: formation of indene and its conversion to naphthalene. Faraday Discuss. 195, 637–670 (2016).
Jasper, A. W. & Hansen, N. Hydrogen-assisted isomerizations of fulvene to benzene and of larger cyclic aromatic hydrocarbons. Proc. Combust. Inst. 34, 279–287 (2013).
Lau, K.-C., Li, W.-K., Ng, C. Y. & Chiu, S.-W. A Gaussian-2 study of isomeric C2H2N and C2H2N+. J. Phys. Chem. A 103, 3330–3335 (1999).
Willacy, K., Allen, M. & Yung, Y. A new astrobiological model of the atmosphere of Titan. Astrophys. J. 829, 79 (2016).
Cui, J. et al. Analysis of Titan’s neutral upper atmosphere from Cassini Ion Neutral Mass Spectrometer measurements. Icarus 200, 581–615 (2009).
Hickson, K. M. et al. The C(3P) + NH3 reaction in interstellar chemistry. II. Low temperature rate constants and modeling of NH, NH2, and NH3 abundances in dense interstellar clouds. Astrophys. J. 812, 107 (2015).
Pope, C. J. & Miller, J. A. Exploring old and new benzene formation pathways in low-pressure premixed flames of aliphatic fuels. Proc. Combust. Inst. 28, 1519–1527 (2000).
Zhao, L. et al. Gas-phase synthesis of benzene via the propargyl radical self-reaction. Sci. Adv. 7, eabf0360 (2021).
Pearce, B. K. D., Ayers, P. W. & Pudritz, R. E. A consistent reduced network for HCN chemistry in early earth and Titan atmospheres: quantum calculations of reaction rate coefficients. J. Phys. Chem. A 123, 1861–1873 (2019).
Pearce, B. K. D., He, C. & Hörst, S. M. An experimental and theoretical investigation of HCN production in the Hadean Earth atmosphere. ACS Earth Space Chem. 6, 2385–2399 (2022).
Marston, G., Nesbitt, F. L., Nava, D. F., Payne, W. A. & Stief, L. J. Temperature dependence of the reaction of nitrogen atoms with methyl radicals. J. Phys. Chem. 93, 5769–5774 (1989).
Marston, G., Nesbitt, F. L. & Stief, L. J. Branching ratios in the N + CH3 reaction: formation of the methylene amidogen (H2CN) radical. J. Chem. Phys. 91, 3483–3491 (1989).
Dobrijevic, M., Loison, J. C., Hickson, K. M. & Gronoff, G. 1D-coupled photochemical model of neutrals, cations and anions in the atmosphere of Titan. Icarus 268, 313–339 (2016).
Loison, J. C., Dobrijevic, M. & Hickson, K. M. The photochemical production of aromatics in the atmosphere of Titan. Icarus 329, 55–71 (2019).
Benne, B., Dobrijevic, M., Cavalié, T., Loison, J.-C. & Hickson, K. M. A photochemical model of Triton’s atmosphere with an uncertainty propagation study. Astron. Astrophys. 667, A169 (2022).
Vanuzzo, G. et al. Reaction N(2D) + CH2CCH2 (allene): an experimental and theoretical investigation and implications for the photochemical models of Titan. ACS Earth Space Chem. 6, 2305–2321 (2022).
Teolis, B. D. et al. A revised sensitivity model for Cassini INMS: Results at Titan. Space Sci. Rev. 190, 47–84 (2015).
Gladstone, G. R. & Young, L. A. New Horizons observations of the atmosphere of Pluto. Annu. Rev. Earth Planet. Sci. 47, 119–140 (2019).
Rap, D. B., Schrauwen, J. G., Marimuthu, A. N., Redlich, B. & Brünken, S. Low-temperature nitrogen-bearing polycyclic aromatic hydrocarbon formation routes validated by infrared spectroscopy. Nat. Astron. 6, 1059–1067 (2022).
McGuire, B. A. et al. Detection of two interstellar polycyclic aromatic hydrocarbons via spectral matched filtering. Science 371, 1265–1269 (2021).
McElroy, D. et al. The UMIST database for astrochemistry 2012. Astron. Astrophys. 550, A36 (2013).
Ohishi, M., McGonagle, D., Irvine, W. M., Yamamoto, S. & Saito, S. Detection of a new interstellar molecule, H2CN. Astrophys. J. 427, L51–L54 (1994).
Thaddeus, P., Vrtilek, J. M. & Gottlieb, C. A. Laboratory and astronomical identification of cyclopropenylidene, C3H2. Astrophys. J. 299, L63–L66 (1985).
Cernicharo, J. et al. Discovery of CH2CHCCH and detection of HCCN, HC4N, CH3CH2CN, and, tentatively, CH3CH2CCH in TMC-1. Astron. Astrophys. 647, L2 (2021).
Tennis, J. D. et al. Detection and modelling of CH3NC in TMC-1. Mon. Not. R. Astron. Soc. 525, 2154–2171 (2023).
Stoks, P. G. & Schwartz, A. W. Basic nitrogen-heterocyclic compounds in the Murchison meteorite. Geochim. Cosmochim. Acta 46, 309–315 (1982).
Sephton, M. A. Organic compounds in carbonaceous meteorites. Nat. Prod. Rep. 19, 292–311 (2002).
Dangi, B. B., Maity, S., Kaiser, R. I. & Mebel, A. M. A combined crossed beam and ab initio investigation of the gas phase reaction of dicarbon molecules (C2; X1Σg+/a3Πu) with propene (C3H6; X1A′): identification of the resonantly stabilized free radicals 1-and 3-vinylpropargyl. J. Phys. Chem. A 117, 11783–11793 (2013).
Werner, H. et al. MOLPRO, version 2015.1, a package of ab initio programs (Univ. Cardiff Chemistry Consultants (UC3), 2015).
Knowles, P. J., Hampel, C. & Werner, H. J. Coupled cluster theory for high spin, open shell reference wave functions. J. Chem. Phys. 99, 5219–5227 (1993).
Dunning, T. H. Jr Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J. Chem. Phys. 90, 1007–1023 (1989).
Peterson, K. A., Adler, T. B. & Werner, H.-J. Systematically convergent basis sets for explicitly correlated wavefunctions: the atoms H, He, B–Ne, and Al–Ar. J. Chem. Phys. 128, 084102 (2008).
Becke, A. D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 98, 5648–5652 (1993).
Lee, C., Yang, W. & Parr, R. G. Development of the Colle–Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 37, 785 (1988).
Frisch, M. J. et al. Gaussian 16, revision C.1 (Gaussian Inc., Wallingford CT, 2016).
Acknowledgements
This work was supported by the US Department of Energy, Basic Energy Sciences, by grant no. DE-FG02-03ER15411 to the University of Hawaii at Manoa. The support of Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), grant nos. 311508/2021-9 and 405524/2021-8, is also acknowledged. We acknowledge fruitful discussions on the fractional abundances of ammonia with C. A. Nixon (NASA Goddard) and K. Willacy (JPL).
Author information
Authors and Affiliations
Contributions
R.I.K. designed the experiments. Z.Y., C.H. and S.J.G. preformed the experiments. A.M.M., P.F.G.V., M.O.A. and B.R.L.G. conducted the electronic structure calculations. J.-C.L, K.M.H. and M.D. conducted the atmospheric modelling of Titan. X.L. performed the astrochemical modelling of TMC-1. Z.Y. and R.I.K. analysed the data and wrote the paper. All authors discussed the data.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Astronomy thanks Ben Pearce and Gianmarco Vanuzzo for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Temperature and energy dependence of the thermal rate constants.
Temperature and energy dependence of the thermal rate constant k(T) and k(E) for the p3 + H in the C + NH3 reaction. TST methods are utilized in the calculation.
Extended Data Fig. 2 Potential energy surfaces of the reaction of dicarbon -ammonia.
Singlet and triplet surfaces of the C2-NH3 system involving different routes to the final products.
Extended Data Fig. 3 Formation pathways to pyridinyl radicals and the pyridine intermediate I.
Distinct pyridinyl radicals and pyridine can be formed from reactions of methylene amidogen (H2CN) with i/n-C4H3 isomers and the cyanomethyl (H2CCN) with propargyl (C3H3).
Extended Data Fig. 4 Formation pathways to pyridinyl radicals and the pyridine intermediate II.
Distinct pyridinyl radicals and pyridine can be formed from reactions of cis-iminomethyl (HCNH) with i/n-C4H3 isomers.
Supplementary information
Supplementary Information
Supplementary Notes 1–4, Figs. 1–3 and Tables 1–4.
Supplementary Data 1
Optimized Cartesian coordinates (Å) and vibrational frequencies (cm−1) for the intermediates, transition states, reactants and products involved in the reactions of C–NH3 and C2–NH3, and the pathways from H2CN·, cis-HCNH and H2CCN· to pyridine and pyridinyl radicals.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, Z., He, C., Goettl, S.J. et al. Low-temperature formation of pyridine and (iso)quinoline via neutral–neutral reactions. Nat Astron (2024). https://doi.org/10.1038/s41550-024-02267-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41550-024-02267-y