Abstract
High fructose corn syrup (HFCS)-associated health problems have raised concerns. We investigated the effects of HFCS-containing drinking water on body fat, intestinal microbiota structure of mice, and the relationships between them. HFCS drinking water significantly increased body fat content and altered the intestinal microbiome. The Christensenellaceae R-7 group negatively correlated with body weight, perirenal fat, epididymal fat, and liver fat percentage.
Similar content being viewed by others
Introduction
High fructose corn syrup (HFCS) has gradually become a prominent form of sugar in the human diet in recent years due to its advantages over sucrose in texture, taste, preparation methods, and cost1. As HFCS is widely used as a sweetener in various beverages and food products, its excessive consumption has become a major health issue. Intake of beverages and food products containing HFCS is associated with changes in systemic and tissue-specific metabolic status, which result in profound effects on the body, such as causing obesity2, insulin resistance-related and obesity-induced non-alcoholic fatty liver disease (NAFLD)3, cardiovascular diseases4, type 2 diabetes mellitus (T2DM)5, reproductive system diseases6, and even cancer7.
The numerous microbes present in the intestinal tracts of humans and mammals regulate many physiological functions, including proteins, fats, and carbohydrates metabolism, the production of vitamins and minerals essential for the body, the maintenance of intestinal integrity and intestinal barrier function, regulation of immune functions, and protection against pathogens. Disturbances to the microbiota in a healthy intestinal tract might result in the onset of various chronic diseases. Intestinal microbiota participates extensively in lipid metabolism and the onset and development of obesity and is associated with metabolic diseases, such as NAFLD, cardiovascular diseases, obesity, T2DM, and metabolic syndrome8.
Current research on the effects of HFCS on humans or animals has mainly focused on metabolic conditions, such as NAFLD and diabetes mellitus, with few studies investigating its effects on gut microbiota changes and composition. Therefore, we employed 16 S rDNA sequencing analysis to determine the effects of HFCS ingestion on intestinal microbiota diversity and composition in mice. By analyzing the correlations between body weight, perirenal fat weight, epididymal fat weight or liver fat percentage, and the colonic microbiota structure, we also deduced the possible mechanisms by which changes in intestinal microbiota structure induce obesity signs.
Results
Effects of HFCS on intestinal microbiota structure and obesity in mice
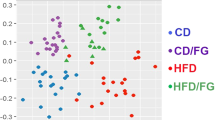
In this study, the long-term intake of drinking water containing 30% HFCS caused significant increases in body weight, perirenal fat, epididymal fat (P = 0.001, P = 0.0009, P = 0.007, respectively), and liver fat percentage in mice (P < 0.0001), as shown by a considerable increase in the proportion of area occupied by positively stained lipid droplets (Fig. 1). Further, high-throughput sequencing revealed the occurrence of significant changes in the colonic microbiota. The community richness of colonic microbiota in the HFCS group was significantly decreased compared with that of the control group (Supplementary Table 1). Beta-diversity analysis revealed the presence of significantly different clusters between the two groups (Supplementary Fig. 1). At the phylum level, the taxonomic distribution of the colonic microbial communities in both groups displayed that Firmicutes, Bacteroidetes, Proteobacteria, Actinobacteria, Tenericutes, and Deferribacteres were (Supplementary Fig. 2a) the dominant bacteria, which accounted for more than 99.29% of the colonic microbiota. Changes in colonic microbiota composition at the genus level were also observed (Supplementary Fig. 2b). In both groups, the top 10 genera with the highest relative abundances were Erysipelotrichaceae_uncultured, Bacteroidales S24-7 group_norank, Allobaculum, Faecalibaculum, Staphylococcus, Lactobacillus, Bacteroides, Lachnospiraceae_uncultured, Turicibacter, and Lachnospiraceae NK4A136. The bacteria with higher relative abundance and significant difference between the two groups were screened and plotted on a heat map (Fig. 2). This indicates that the intake of HFCS-containing drinking water resulted in considerable changes in the colonic microbiota structure of mice.
Correlations between microbial genera and obesity indices
From the 42 genera with significant differences in relative abundance between the two groups, the obesity-associated genera Christensenellaceae R-7 group and Tyzzerella, as well as the pathogenic bacteria Erysipelatoclostridium and Helicobacter, were selected to analyze their correlations with phenotypic variables of body weight, perirenal fat, epididymal fat, and liver fat percentage (Supplementary Fig. 3). We found that Tyzzerella, Erysipelatoclostridium, and Helicobacter were positively correlated with body weight, perirenal fat, epididymal fat, and liver fat percentage, whereas Christensenellaceae R-7 group was strongly negatively correlated. The genus Tyzzerella of the family Lachnospiraceae showed a significant increase in abundance after intervention with HFCS-containing drinking water. At the genus level, the relative abundances of conditional pathogenic bacteria, such as Erysipelatoclostridium and Helicobacter, were significantly increased in the HFCS group compared with those in the control group.
Discussion
HFCS may induce obesity by affecting Christensenellaceae R-7 group in intestinal flora
The increase in body weight and visceral fat was consistent with previous studies9,10. The accumulation of visceral fat leads to a higher tendency of insulin resistance, disorders in sugar utilization, and increased lipolysis, which are key factors for the onset of metabolic syndrome. Therefore, the measurement and analysis of body weight, visceral fat content, and peripheral fat content are of great importance11. Wostmann et al. found that the increase in body weight of mice in a germ-free environment was slower than that of normal mice, which demonstrates the beneficial effects of microbial communities on fat deposition12. Other researchers have also directly demonstrated the relationships between microbiota and metabolic disorders, such as obesity13. In mice on a high-fat diet and obese individuals reportedly have higher abundances of Firmicutes and Bacteroidetes in the body, forming an obesity-type intestinal microbiota14, but other studies have reported that a high-fat diet reduces the ratio of Firmicutes to Bacteroidetes and the abundances of intestinal microbes15. The results of the present study might be attributed to the fact that mice of the HFCS group were only provided HFCS-containing drinking water and basal feed without the addition of high-fat feed. Firmicutes are capable of utilizing carbohydrates in food for the synthesis of butyrate, which serves as an energy source and maintains epithelial cell morphology and normal function in the colon16. Pediatric researchers at the University of Alberta in Canada found that Lachnospiraceae species feed on undigested carbohydrate fibers in the human body, thereby contributing additional calories through carbohydrate degradation and resulting in obesity13. Christensenellaceae, a member of the phylum Firmicutes, is abundant in the colons of humans and animals and accounts for 0.01% of the fecal microbial communities17. Christensenellaceae are also closely associated with host health as their relative abundance is associated with the onset of colorectal cancer and inflammatory bowel disease18,19,20,21. Further, the relative abundance of this family in the intestines is associated with body mass index, a direct indicator of obesity, in the populations of many countries, which includes adult men and women of different age groups17. The existence of a negative correlation between Christensenellaceae and the amount of visceral fat has also been reported22. Metabolic disorders are usually related to dietary patterns, with research evidence indicating that Christensenellaceae R-7 group is decreased in populations on a refined sugar diet23. Such evidence is consistent with the results of the present study, which showed that long-term high-dose HFCS consumption led to a decrease in Christensenellaceae R-7 group, thereby inducing obesity or even the inflammatory response in mice. These results are consistent with those reported by Beaumont et al17,22. and demonstrate that the relative abundance of Christensenellaceae R-7 group is negatively correlated with body weight and visceral fat content. Therefore, it can be deduced that HFCS intake induces obesity in mice by regulating the relative abundance of the genus Christensenellaceae R-7 group.
The results of this study provide theoretical support for the rational use of sweeteners, suggesting that HFCS may induce obesity by affecting Christensenellaceae R-7 group in intestinal flora. However, the specific mechanism is still unclear, which will be further studied in the follow-up study.
Methods
Sixteen male specific pathogen-free grade C57BL/6J mice aged 3 weeks and weighing 18–22 g each were purchased from Shanghai SLAC Laboratory Animal Co., Ltd. (Shanghai, China) [License No. SCXK(Lu)2017-0005]. The study was approved by the Zhejiang Provincial Ethics Committee for Laboratory Animals (Ethical Approval No. 78865576).
The mice were randomly divided into the control group and HFCS group (8 each) after one week of acclimatization. All animals were reared in a clean environment at the Laboratory Animal Center of Zhejiang Academy of Agricultural Sciences (Animal Experimentation License No. 286868667) under the following conditions: rearing temperature, 21 ± 2 °C; relative humidity, 50–80%; 12-h light/dark cycle, and ad libitum access to food and water. Co60-irradiated normal feed (18.6% crude protein (mass %), 4.8% crude fat, 61% carbohydrate) was purchased from Jiangsu Xietong Pharmaceutical Bio-engineering Co., Ltd. (Jiangsu, China). HFCS (F55, i.e., 55% fructose and 45% glucose) was purchased from COFCO (Chengdu) Grains and Oils Industries Co., Ltd. (Sichuan, China).
The control and HFCS groups were given pure water and 30% (w/v) HFCS24, respectively, in addition to the normal diet. After 16 weeks of rearing, the mice were anesthetized and dissected. The colon of each mouse was removed, and the colonic contents were collected into a sterile centrifuge tube and stored in a − 80 °C freezer prior to intestinal microbiota analysis. The liver segments were fixed in 4% paraformaldehyde, and then frozen slices were made. The slices were stained with Oil Red solution (Servicebio) and examined under microscope. Genomic DNA was extracted from the colonic contents using the QIAamp DNA Stool Mini Kit (QIAGEN, Valencia, CA, USA) and confirmed by 1% agarose gel electrophoresis. Based on the specified sequencing regions, specific barcoded primers were synthesized for the amplification of the V3 + V4 regions of the 16S rDNA gene. The agarose gel was sliced to recover the amplification products, which were quantified using the QuantiFluor™ system and sequenced using the HiSeq 2500 PE250 platform at Mingke Biotechnology (Hangzhou) Co., Ltd after purification. Raw sequencing data were subjected to raw data filtering, tag assembly, tag filtering, and the removal of chimeric tags to eliminate low-quality data and obtain effective tags for subsequent analysis. Operational classification unit (OTU) analysis was performed on non-repeated sequences according to 97% similarity. Species matching was performed for all representative sequences of OTU using RPD databases. Principal component analysis was used to analyze beta diversity among samples. Pie charts are used to show taxa at the phylum and genus levels.
All experimental data were statistically analyzed using SPSS 26.0 and plotted using GraphPad. Differences were compared using the t-test and results were expressed as the mean and standard error of the mean; * denotes P < 0.05, ** denotes P < 0.01, and *** denotes P < 0.001.
Data availability
The authors declare that the data supporting the findings of this study are presented within the manuscript. The sequences obtained in the study were deposited in the NCBI Sequence Read Archive under accession number PRJNA 744020. Additional data sources are also available from the corresponding author upon reasonable request.
References
Yang, H. Characteristics and application of high fructose corn syrup. Food Sci. 23, 137–139 (2002).
Bray, G. A. & Popkin, B. M. Dietary sugar and body weight: have we reached a crisis in the epidemic of obesity and diabetes?: health be damned! Pour on the sugar. Diabetes Care 37, 950–956 (2014).
Mock, K., Lateef, S., Benedito, V. A. & Tou, J. C. High-fructose corn syrup-55 consumption alters hepatic lipid metabolism and promotes triglyceride accumulation. J. Nutr. Biochem. 39, 32–39 (2017).
Hieronimus, B. et al. Synergistic effects of fructose and glucose on lipoprotein risk factors for cardiovascular disease in young adults. Metabolism 112, 154356 (2020).
Cozma, A. I. & Sievenpiper, J. L. The role of fructose, sucrose and high-fructose corn syrup in diabetes. Eur. Endocrinol. 10, 51–60 (2014).
Aslankoc, R. & Ozmen, O. The effects of high‐fructose corn syrup consumption on testis physiopathology—the ameliorative role of melatonin. Andrologia 51, e13327 (2019).
Strober, J. W. & Brady, M. J. Dietary fructose consumption and triple-negative breast cancer incidence. Front. Endocrinol. 10, 367 (2019).
Fan, Y. & Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 19, 55–71 (2021).
Ou, H. P., Luo, Z. Z., Fan, Y. & Yao, X. P. Effects of short-term addition of high fructose corn syrup on body weight and liver function indexes in mice. Prog. Vet. Med. 38, 66–69 (2017).
Bocarsly, M. E., Powell, E. S., Avena, N. M. & Hoebel, B. G. High-fructose corn syrup causes characteristics of obesity in rats: increased body weight, body fat and triglyceride levels. Pharmacol. Biochem. Behav. 97, 101–106 (2010).
Tchernof, A. & Després, J. P. Pathophysiology of human visceral obesity: an update. Physiol. Rev. 93, 359–404 (2013).
Wostmann, B. S., Larkin, C., Moriarty, A. & Bruckner-Kardoss, E. Dietary intake, energy metabolism, and excretory losses of adult male germfree Wistar rats. Lab. Anim. Sci. 33, 46–50 (1983).
Tilg, H., Zmora, N., Adolph, T. E. & Elinav, E. The intestinal microbiota fuelling metabolic inflammation. Nat. Rev. Immunol. 20, 40–54 (2020).
Turnbaugh, P. J. et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444, 1027–1031 (2006).
Li, W. F. et al. Polyphenol-rich loquat fruit extract prevents fructose-induced nonalcoholic fatty liver disease by modulating glycometabolism, lipometabolism, oxidative stress, inflammation, the intestinal barrier and the gut microbiota in mice. J. Agr. Food Chem. 67, 7726–7737 (2019).
Xu, Z. Y. et al. Research progress on the regulation of intestinal microecology by butyric acid producing bacteria and the intervention effect of traditional Chinese medicine. Chin. J. Etmf. 26, 226–233 (2020).
Goodrich, J. et al. Human genetics shape the gut microbiome. Cell 159, 789–799 (2014).
Morotomi, M., Nagai, F. & Watanabe, Y. Description of Christensenella minuta gen. nov., sp. nov., isolated from human faeces, which forms a distinct branch in the order Clostridiales, and proposal of Christensenellaceae fam. nov. Int. J. Syst. Evol. Micr. 62, 144–149 (2012).
Waters, J. L. & Ley, R. E. The human gut bacteria Christensenellaceae are widespread, heritable, and associated with health. BMC Biol. 17, 83 (2019).
Yazici, C. et al. Race-dependent association of sulfidogenic bacteria with colorectal cancer. Gut 66, 1983–1994 (2017).
Leonardo, M. et al. Identification of universal gut microbial biomarkers of common human intestinal diseases by meta-analysis. Fems Microbiol. Ecol. 93, 1–12 (2017).
Beaumont, M. et al. Heritable components of the human fecal microbiome are associated with visceral fat. Genome Biol. 17, 189 (2016).
Gertraud, M. et al. Fecal microbial diversity and structure are associated with diet quality in the multiethnic cohort adiposity phenotype study. J. Nutr. 149, 1575–1584 (2019).
Bhat, S. F. et al. Exposure to high fructose corn syrup during adolescence in the mouse alters hepatic metabolism and the microbiome in a sex-specific manner. J. Physiol. 599, 1487–1511 (2021).
Acknowledgements
We would like to thank State Key Laboratory for Managing Biotic and Chemical Threats to the Quality and Safety of Agroproducts, Grant/Award Number: 2010DS700124-ZZ2017.
Author information
Authors and Affiliations
Contributions
X.W. and J.L. designed the experiment. X.W. conducted the animal experiments. X.W., R.H., and J.L. wrote and revised the manuscript. X.W., R.H., and J.L. performed experimental analysis, collected, and analyzed the data. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, X., Zhu, L., Li, X. et al. Effects of high fructose corn syrup on intestinal microbiota structure and obesity in mice. npj Sci Food 6, 17 (2022). https://doi.org/10.1038/s41538-022-00133-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41538-022-00133-7
This article is cited by
-
Assessing the impact of three feeding stages on rumen bacterial community and physiological characteristics of Japanese Black cattle
Scientific Reports (2024)
-
The number of metabolic syndrome risk factors predicts alterations in gut microbiota in Chinese children from the Huantai study
BMC Pediatrics (2023)
-
Persistent and Unsuccessful Attempts to Cut Down on Ultra-Processed Foods and the Associated Challenges for Dietary Adherence
Current Addiction Reports (2022)