Abstract
Cognitive impairments are a core and persistent characteristic of schizophrenia with implications for daily functioning. These show only limited response to antipsychotic treatment and their neural basis is not well characterised. Previous studies point to relationships between cortical thickness and cognitive performance in fronto-temporal brain regions in schizophrenia patients (SZH). There is also evidence that these relationships might be independent of symptom severity, suggesting dissociable disease processes. We set out to explore these possibilities in a sample of 70 SZH and 72 age and gender-matched healthy controls (provided by the Center of Biomedical Research Excellence (COBRE)). Cortical thickness within fronto-temporal regions implicated by previous work was considered in relation to performance across various cognitive domains (from the MATRICS Cognitive Battery). Compared to controls, SZH had thinner cortices across most fronto-temporal regions and significantly lower performance on all cognitive domains. Robust relationships with cortical thickness were found: visual learning and attention performance correlated with bilateral superior and middle frontal thickness in SZH only. Correlations between attention performance and right transverse temporal thickness were also specific to SZH. Findings point to the importance of these regions for cognitive performance in SZH, possibly reflecting compensatory processes and/or aberrant connectivity. No links to symptom severity were observed in these regions, suggesting these relationships are dissociable from underlying psychotic symptomology. Findings enhance understanding of the brain structural underpinnings and possible aetiology of cognitive impairment in SZH.
Similar content being viewed by others
Introduction
Schizophrenia is a complex disorder that includes widespread neurocognitive1,2 and neuroanatomical impairments3,4. Studies have shown that compared to healthy controls, schizophrenia patients (SZH) have impaired cognitive performance across all cognitive domains including processing speed5, attention and vigilance6, working memory7, verbal learning8, visual learning9, reasoning/problem solving9, and social cognition10. Impaired functioning is a hallmark of SZH and cognitive deficits have been shown to be closely tied to functional outcome in SZH11,12,13. However, cognitive impairments are largely unresponsive to pharmacological therapy14. These cognitive deficits are also observed in first episode psychosis samples suggesting they are not due to exposure to neuroleptic medication13,15. Cognitive training also has limited efficacy in certain domains. SZH show very limited response to perceptual training, for example, and while executive function training does have positive effects on neurocognition and functioning, these take several months to become apparent16. Developing a better understanding of the neuroanatomical basis of cognitive impairment in SZH could explain why this is so, and help inform treatment approaches.
Reduced cortical thickness has been consistently demonstrated in SZH17, including first episode psychosis18. The meta-analysis by van Erp et al.17, found evidence for widespread cortical thinning; effect sizes were largest in frontal and temporal regions. Strong cortico-cortical connectivity between affected regions has been shown and thus thinning appears to occur within an interconnected network19. While seemingly relatively stable and not linked to the duration of illness20,21, these morphological abnormalities have been linked to symptomology albeit inconsistently. Associations between positive symptom severity and thinning in frontal and temporal regions have been identified in SZH22,23 and first episode patients21; associations with negative symptoms seem less reliable. Abnormalities also link to the degree of cognitive impairment. A cognitively relatively intact subgroup of SZH seems to exist24 with less pronounced cortical thinning25,26; conversely, clustering patients according to cortical thickness patterns differentiate those with greater impairment27. The degree to which such neural correlates are attributable to cognitive impairment or to psychotic processes remains unclear, however. Some studies suggest that cognitively near-normal SZH show minimal cortical thinning compared to controls25,28 while others suggest otherwise29,30. Thus the role and significance of cortical thinning remain uncertain. Clarifying whether it associates mostly with cognitive impairment, or is attributable to a more general disease process, is important. If thinning relates specifically to cognitive impairment but not symptomology, this dissociability would point to separate disease pathologies and suggest that the core neurobiology of psychosis lies elsewhere, such as in subcortical nuclei31 and/or white matter pathways32. Thus, disentangling these relationships is important to enhance knowledge of the underlying neurobiology.
Studies into cognition-thickness relationships are informative in this regard. Limited data is available, but studies suggest relationships that are absent and/or specific to SZH within specific fronto-temporal regions29,33,34. Across various cognitive domains, Hanford et al.29 found cognition-thickness relationships in left superior temporal and right middle frontal that were present in controls but lacking in SZH, whereas left superior frontal thickness correlated positively with cognitive performance in SZH only, possibly indicating compensatory processes. Frontal cortex was also implicated by Oertel-Knöchel et al.35 who found cortical thickness in bilateral inferior frontal gyrus correlated with subjective cognitive dysfunction in SZH, but not in controls.
Temporal effects have also been shown. Hartberg et al.34 identified correlations between processing speed and thickness in left middle temporal and left transverse temporal that were present in SZH but not controls. Ehrlich et al.36 found that working memory performance in SZH correlated with thickness in right middle and right superior temporal gyrus (STG), rather than lateral prefrontal cortex as seen in controls. Edgar et al.33 focused only on cortical thickness in bilateral STG and prefrontal cortex: in SZH (but not in controls), thinning in left and right STG was associated with poorer attention performance on the Continuous Performance Test. STG included transverse temporal in these latter 2 studies. Finally, Pan et al.27 employed a clustering approach to categorise SZH with pronounced cortical thinning; this was evident across fronto-temporal regions, and this group showed greater working memory impairment.
Although studies are few and results are somewhat heterogeneous, evidence does suggest that there exist cognition-thickness relationships within temporal and frontal regions that are present in SZH but not in controls. However, some of the aforementioned studies only included a restricted range of cognitive domains. Here, we investigated links between cortical thickness and cognition across a broad range of cognitive domains using the standardised MCCB task battery, in a sample of seventy patients and controls. While Hanford et al.29 was also comprehensive in the MCCB domains included, the sample size employed here is larger. Whether cognitive-thickness relationships are dissociable from the degree of positive and negative symptomology also remains uncertain as few studies have explored this explicitly: here we compared correlations between thickness, cognition, and symptoms to address this question. Thus the current study aimed to better characterise relationships between cortical thickness and cognitive performance in SZH, and also interrogate whether these relationships are dissociable from links to symptom severity.
First, to characterise differences between SZH and controls, we assessed between-group differences in cognitive performance in six key domains (attention, working memory, reasoning/problem solving, processing speed, visual learning, and verbal learning). Overall composite cognitive scores were also considered. All measures were drawn from the Measurement and Treatment Research to Improve Cognition (MATRICS) Consensus Cognitive Battery. Cortical thickness within the regions of interest (ROIs, comprising regions implicated by previous studies) was also compared between-groups. Associations between domain scores and thickness were then assessed separately for SZH and controls, and correlations were compared between-groups to identify abnormal structure-function relationships in SZH. Associations between thickness and symptomology were also assessed in SZH to assess whether thickness within the ROIs was associated with symptom severity. Also, regression models investigated the extent to which variance in cognitive performance could be explained by symptomology, medication use, and other relevant variables. Based on previous findings we hypothesized lower cognitive performance on all cognitive domains, and thinner cortex in all ROIs, in SZH compared to healthy controls. We also expected to see thickness-cognition relationships in SZH that differ significantly from those present in healthy controls. Finally, we expected these thickness-cognition relationships in SZH to be at least partially dissociable from relationships between thickness and symptomology.
Results
Demographic data of the study sample
Means and standard deviations for all demographic data are reported in Table 1. No statistically significant differences were observed between SZH and healthy control groups on age and gender (all p values > 0.05). Healthy controls had a significantly higher level of education (M = 13.78, SD = 1.55) compared to SZH (M = 12.71, SD = 1.78, p < 0.001).
Neurocognitive performance of the study sample
Means and standard deviations for all MATRICS domain scores are reported in Table 2. ANCOVAs (age, gender, and education as covariates) showed that SZH had significantly lower performance on all MATRICS domain scores. All of these differences remained significant after correction for multiple comparisons (p < 0.05, FDR corrected).
MRI–Cortical thickness differences between SZH and controls
Between-group cortical thickness differences were assessed using ANCOVA with age and gender as covariates.
After FDR correction for multiple comparisons, thinner cortices were observed in SZH within superior frontal (bilateral), middle frontal, inferior frontal, superior temporal (bilateral), and middle temporal (p < .05, FDR corrected) (Table 3). For between-group differences in other brain regions that were not included in our ROIs, see Supplementary Table 5.
Multiple regression analysis
Overall, only PANSS negative and medication use explained variance in cognitive performance, duration of illness, gender, PANSS positive, and education were not significant predictors in any of the models. PANSS negative (p = 0.002) and medication (p = 0.021) were significant predictors of processing speed. Medication use was the only predictor of attention/vigilance scores (p = 0.040). PANSS negative score was the only predictor of reasoning/problem-solving scores (p = 0.021). Models did not explain significant variance in working memory or visual memory performance. PANSS negative (p = 0.011) and medication (p = 0.031) were significant predictors of overall composite scores (Supplementary Table 1).
Correlations between MATRICS domain scores and cortical thickness
Partial correlation analyses (controlling for age and education) investigated relationships between neurocognitive performance and cortical thickness within the ROIs. Correlations were assessed separately for each group and compared using a percentile bootstrap method.
Processing speed: scores in SZH positively correlated with right transverse temporal thickness (uncorrected p = 0.023) although this was not significant after FDR correction. No associations were seen in healthy controls (all p values > 0.05) (Table 4). A significant difference in correlations between groups was identified for this region, as the confidence intervals for the bootstrap distribution of the differences between correlation coefficients did not overlap with zero (95% CI: 0.29, 0.41).
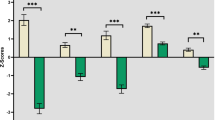
Attention and Vigilance: Scores in SZH positively correlated with thickness in left and right superior frontal (see Figs. 1a, 1b) and right transverse temporal thickness (see Fig. 1c), and these relationships were significant after FDR correction. A positive correlation with middle frontal was observed in SZH but was only present at trend (p = 0.054) after FDR correction. A positive correlation with right superior temporal was observed in SZH (uncorrected p = 0.044) but did not survive FDR correction. No associations were seen in healthy controls (Table 4). Between-groups, significant differences in correlations was found for left (95% CI: 0.32, 0.43), and right (95% CI: 0.34, 0.45) superior frontal, and right transverse temporal (95% CI: 0.25, 0.37).
Scatter plots demonstrating significant positive relationships between cognitive domains and cortical thickness (mm), adjusted for age and gender, in SZH and controls. a Larger cortical thickness in right superior frontal related to the better attention/vigilance performance in SZH (r = 0.311, p = 0.010). b Larger thickness in left superior frontal related to the better attention/vigilance performance in SZH (r = 0.321, p = 0.008). c Larger thickness in right transverse temporal thickness related to the better attention/vigilance performance in SZH (r = 0.333, p = 0.006). d Larger thickness in right superior frontal positively correlated with better visual learning performance in SZH (r = 0.305, p = 0.011). e Larger left superior frontal positively correlated with better visual learning performance in SZH (r = 0.339, p = 0.011). f Larger middle frontal thickness positively correlated with better visual learning performance in SZH (r = 0.322, p = 0.007). g Larger right superior temporal thickness positively correlated with visual learning in healthy controls (r = 0.423, p < 0.001).
Visual learning: scores in SZH positively correlated with thickness in left and right superior frontal (see Fig. 1d, 1e) and middle frontal (see Fig. 1f) and these were significant after FDR correction. A relationship with middle temporal was observed (p = 0.039) which did not survive FDR correction; a strong uncorrected trend (p = 0.053) was seen in left transverse temporal. In controls, visual learning scores positively correlated with thickness in right superior temporal (see Fig. 1g) which survived FDR correction, significant uncorrected relationships were present in left superior and middle temporal although were only seen at trend-level after FDR-correction (Table 5). No difference in correlations between groups was found for any of these regions.
Working memory: No associations were seen either in healthy controls or SZH (Supplementary Table 2).
Reasoning/Problem Solving: No associations were seen either in healthy controls or SZH (Supplementary Table 2).
Verbal learning: No associations were seen either in healthy controls or SZH (Supplementary Table 3).
Composite scores: In SZH, there was evidence of a positive relationship between composite scores and thickness in right transverse temporal (p = 0.035) although this did not survive FDR correction. Strong uncorrected positive trends were seen in middle frontal (p = 0.054) and left superior frontal (p = 0.054). No associations were seen in healthy controls (Table 5). Between-group differences in correlations were observed for right transverse temporal (95% CI: 0.25, 0.37).
Correlations between symptom severity and cortical thickness
Partial correlation analyses (controlling for age, education, and olanzapine equivalent) were performed to investigate relationships between symptom severity and cortical thickness within the ROIs.
Correlates of positive symptoms: Negative correlations were observed with inferior frontal thickness (uncorrected p = 0.017) and left superior temporal thickness (uncorrected p = 0.041), although these were not significant after FDR correction. No other associations were observed (Supplementary Table 4).
Correlates of negative symptoms: No associations were observed (Supplementary Table 4).
Discussion
The present study set out to investigate whether cortical thickness within frontal and temporal brain regions correlates with neurocognitive performance in SZH, whether these relationships differed from those of a healthy control group with similar age and gender composition, and whether these relationships were dissociable from those linked to symptom severity. As expected, significant group differences in cognitive performance were found with SZH showing poorer performance in all cognitive domains under study. The level of medication use explained significant variance in cognitive performance, in line with previous results52.
ROIs were selected based on previous work suggesting structure-function relationships particular to SZH might be present within specific fronto-temporal regions. As expected, significantly thinner cortex within the ROIs were evident in SZH, in superior, middle, and inferior frontal, and superior and middle temporal regions (but not transverse temporal) while controlling for age and gender. This is in line with the previous literature53,54. We focused on cortical thickness since brain volume measures are influenced by cortical surface area as well as thickness. Generally, surface area in SZH shows fewer links to symptomology and differs less against control groups23. Cortical thickness specifically reflects the density and size of neuronal cells and neuropil (axons + dendrites + glia)55,56. Postmortem studies in SZH point to reduced density of neuronal cells and neuropil, while the actual number of neurons appears to be unaffected57,58; cortical thickness measures might thus be more sensitive than volumetric comparisons.
In terms of links between symptom severity and thickness within the ROIs, positive symptoms correlated with thickness in inferior frontal and left superior temporal regions, but these did not survive FDR correction. Reduced inferior frontal volume23,59 and reduced bilateral superior temporal thickness60 have previously been linked to positive symptoms severity in SZH. No associations with negative symptoms were seen, in contrast to a large recent meta-analysis which pointed to associations in prefrontal regions61. However, links to negative symptoms are less robust in the literature, possibly reflecting a relatively greater contribution of other neural parameters and social influences to negative symptomology23.
Our main aim was to examine between-group differences in associations between cortical thickness and cognitive performance. Results revealed some robust relationships in SZH that were absent in controls. Better performance in attention/vigilance associated with thicker right transverse temporal and bilateral superior frontal in SZH, but not in controls. Processing speed scores in SZH also positively correlated with right transverse temporal thickness: although this did not survive FDR correction, the difference in correlations between groups was significant. Visual learning associated with thicker bilateral superior and middle frontal in SZH, in controls associations were seen with right superior temporal thickness but no between-group differences in correlations were found for this cognitive domain. No relationships were seen for working memory or reasoning/problem-solving. On the composite score, there was evidence of an (uncorrected) positive relationship in SZH with thickness in right transverse temporal; a significant between-group difference in correlations was observed for this region. Overall, results pointed to abnormal structure-function relations in SZH in superior frontal and right transverse temporal regions.
Attention/vigilance performance correlated with superior frontal cortex thickness in SZH only. Superior frontal cortex was seen to be thinner in the SZH group overall, when compared to controls. Reduced pyramidal layer thickness seems to be the major contributor to decreased frontal lobe thickness in SZH62. Pyramidal neurons function as output cells; loss of these likely affects intra- and inter-region connectivity with implications for advanced cognitive functioning. Aberrant pruning processes during development63 and reduced blood flow in the prefrontal cortex62 are likely contributing factors. Hanford et al.29 observed a positive relationship between superior frontal thickness and overall cognitive performance, in SZH only (also using the MATRICS battery, but in SZH/control groups that were matched on cognitive ability). Hanford et al.29 suggest this could reflect operation of compensatory processes in higher-performing SZH. Furthermore, enhanced functional connectivity of the left superior frontal gyrus has been put forward as a potential phenotypic marker for the disease: Ding et al.64 found increased functional connectivity specific to the left superior frontal gyrus in both drug-naive SZH and their unaffected siblings; connectivity values of this region could differentiate patients and siblings from controls with good sensitivity, and values did not correlate with symptom severity or illness duration suggesting it could represent a trait marker. However, Ding et al.64 did not assess links with cognition; the current findings suggest this is an important avenue to be explored.
Results pointed to a relationship between right transverse temporal thickness and attention/vigilance; relationships between right transverse temporal thickness, processing speed, and composite scores were present at an uncorrected threshold. These correlations were found to be significantly different between groups, suggesting SZH-specific relationships between right transverse temporal thickness and cognition. Previous studies identified relationships between thickness of right STG (incorporating transverse temporal) and attention33, and verbal processing/working memory recall36, these relationships were absent in controls and present bilaterally in Edgar et al.33. Hartberg et al.34 found a positive relationship between left transverse temporal thickness and processing speed only in SZH and not controls. Taken together, findings confirm the particular importance of temporal lobe thickness (and possibly transverse regions in particular) for cognitive function in SZH across multiple domains. There are various possibilities that could explain this. Firstly, decreased or reversed anatomical asymmetry (particularly in the temporal lobe) has been reported in SZH65 which could potentially contribute33, although here we found overall between-group differences were present in left and right superior, but not transverse, temporal cortices. Alternatively (or in addition) SZH could be engaging a larger network of cortical regions to compensate for prefrontal cortical dysfunction66. Altered connectivity and fronto-temporal network dysfunction (as discussed above) could be of importance in this. Given that we found SZH-specific relationships for attention/vigilance performance with both right transverse temporal and bilateral superior frontal thickness, this explanation is supported by the data. Further investigations, linking the findings reported here to aberrant fronto-temporal connectivity patterns, would be worthwhile.
Also worth noting are previous findings showing that volume loss in transverse temporal regions (Heschl’s gyrus, primary auditory cortex) correlates with the severity of auditory hallucinations in SZH. This correlation seems to be more robust with left transverse temporal67,68 but right-lateralised associations have also been reported68,69. More broadly, positive symptom severity has been seen to correlate with STG thinning in both hemispheres61 and could, therefore, be a contributing factor to structure-cognition relationships in STG70.
In the current study, regions showing links to cognitive performance did not overlap with those showing links to symptom severity. Indeed, although we found some association between positive symptoms and thinning in inferior frontal and left superior temporal regions, these did not survive multiple comparisons correction. This contrasts with the statistically robust relationships between thickness and cognition identified in the SZH group. This supports the suggestion that structure-cognition relationships in SZH are dissociable from symptomology, pointing to independent processes. Furthermore, regression models showed that PANSS positive symptoms did not explain variance in any cognitive domain, although PANSS negative did explain significant variance in processing speed, reasoning/problem solving, and overall composite scores. However, we cannot discount the possibility that the present study lacked the sensitivity to detect relationships with symptoms. Although larger than many previous investigations into structure-cognition relationships, the sample size was relatively modest compared to recent studies investigating links to symptoms: a greater number of participants might have led to the detection of more subtle differences. Moreover, even though the study sample well-matched on age and gender, lower levels of education in the SZH group might have affected results. However, we adjusted all analyses for education, and unmatched education level is common in the literature6,33,71,72. Sensitivity of the measures used might also be an issue. Previous work into structure-cognitive function relationships in SZH have yielded inconsistent results, and not all studies have found evidence that relationships differ between SZH and controls (e.g., Hartberg et al.72). We did not find any relationships between cortical thickness and reasoning/problem solving or working memory (in contrast with Ehrlich et al.36, who used different memory tasks): choice of methodology might be a factor. Other neuropsychological tests might be more sensitive to relationships between cortical structures and performance in these functional domains in SZH, and this is also true for the symptom measures. This is exemplified by Oertel-Knöchel et al.35 who found no relation between cortical thinning and PANSS scores, but a correlation between left STG thinning and hallucinations was revealed when the Revised Hallucination Scale was used instead. Also, some reports point to a tighter coupling between hippocampus volume and memory performance in SZH compared to controls36 but subcortical structures were not included in the present investigation. Finally, recent work has shown that global fractional anisotropy73 and genomic74,75,76,77 contributions to cognition overlap substantially between SZH and controls. The SZH-specific thickness-cognition relationships identified here should be considered, and explored further, in this context.
In conclusion, this study provides important details regarding cortical thickness-cognition links in SZH which remains an important but understudied field of investigation. SZH showed widespread cortical thinning across most of the fronto-temporal regions under study, and significantly impaired performance across all cognitive domains. Based on a highly reliable and validated cognitive battery, cortical thickness in bilateral superior frontal and right transverse temporal regions correlated positively with cognitive performance (particularly attention/vigilance) in patients but not controls. These findings accord with previous results suggesting that thickness in these regions are of specific importance for cognitive performance in SZH, possibly reflecting compensatory processes. On the other hand, no links to symptom severity were present in these regions, suggesting the structural underpinnings of cognitive deficits might be somewhat dissociable from that of symptom severity and could reflect separate disease processes. Delineating the neural bases of each are of importance in developing our understanding of the aetiology of this complex disease.
Methods
Subjects
The data for 70 SZH (18 females, aged between 19 and 65, mean age 37.56, see Table 1) and 72 age and gender-matched healthy controls (18 females, aged between 18 and 65, mean age 38.44, see Table 1) from the publicly-available dataset provided by the Center of Biomedical Research Excellence (COBRE, http://fcon_1000.projects.nitrc.org/indi/retro/cobre.html)37,38,39,40 were used for this study. The Positive and Negative Syndrome Scale (PANSS)41 was used to assess symptoms. Written informed consent was obtained before participation under the guidelines of the Institutional Review Board at the University New Mexico (UNM)42. All patients were receiving antipsychotic medication: doses at the time of MRI scan were converted to olanzapine equivalents (see Table 1).
Cognitive scores were screened and individuals with outliers in any domain were excluded based on Interquartile Range (IQR): criteria for exclusion were values below Q1-1.5 IQR or above Q3 + 1.5 IQR, box-and-whisker plots were used78,79 (also known as Tukey’s method80,81). After removing individuals with outliers the final sample consisted of 70 SZH (18 females, aged between 19 and 65, mean age 37.56, see Table 1) and 72 age and gender-matched healthy controls (18 females, aged between 18 and 65, mean age 38.44, see Table 1). Schizophrenia was diagnosed by a psychiatrist using the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, Fourth Revision (DSM-IV)82. The exclusion criteria were defined by the research centre as intellectually disability, head trauma with more than 5 minutes loss of consciousness, history of substance abuse or dependence within the previous 12 months, or neurological disorder.
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
MRI acquisition
The COBRE group collected raw structural imaging data with a Siemens TIM Trio 3 T scanner (Siemens Erlangen, Germany) using a 12-channel head coil. The details of the procedure are described elsewhere43,44, also see http://schizconnect.org/documentation#data_models. High-resolution T1-weighted structural images were acquired using an MPRAGE sequence with the following parameters: TR/TE/TI = 2530/[1.64, 3.5, 5.36, 7.22, 9.08]/900 ms, flip angle = 7°, FOV = 256 × 256 mm, Slab thickness = 176 mm, Matrix = 256x256x176, Voxel size =1x1x1 mm, Number of echos = 5, Pixel bandwidth =650 Hz, Total scan time = 6 min. All images were visually inspected, and then cortical reconstruction was performed using the Freesurfer 6.0.0 image analysis suite (http://surfer.nmr.mgh.harvard.edu). The cortical parcellation was based on the Destrieux atlas45.
The reconstruction pipeline employed by FreeSurfer includes intensity normalization, motion correction, and the exclusion of non-brain tissue was performed using a hybrid watershed/surface deformation procedure. Images are transformed to Talairach space and the subcortical white matter and deep grey matter structures are segmented83,84. Cortical thickness measurements were calculated using FreeSurfer’s ‘recon -all’ pipeline which calculates the average distance between the pial surface and the white/grey matter boundary for each region85,86.
Cognitive measures
All participants completed the MATRICS (http://www.matricsinc.org/) battery which includes seven cognitive domains previously shown to be impaired in SZH, namely, working memory, attention/vigilance, verbal learning and memory, visual learning and memory, reasoning and problem solving, speed of processing, and social cognition46,47,48,49. Scores for each domain as well as a composite score -an average of the t-scores from cognitive domains- were converted to standardized T-scores by the COBRE group and our analyses were based on these. The duration of administration is ~70 min per person. Domain scores are a composite based on a variety of independent tests with satisfactory validity and reliability47. The current study considered MATRICS total scores, and scores from the following domains. The individual tests make up these domains are:
-
Speed of Processing: Brief Assessment of Cognition in Schizophrenia (BACS): Symbol-Coding; Category Fluency test: Animal Naming, Trail Making Test: Part A (TMT-A),
-
Attention/Vigilance: Continuous Performance Test-Identical Pairs (CPT-IP)
-
Working Memory: Wechsler Memory Scale, 3rd Edition (WMS-III): Spatial Span, Letter-Number Sequencing (LNS)
-
Visual Learning: Brief Visuospatial Memory Test-Revised (BVMT-R)
-
Reasoning and Problem Solving: Neuropsychological Assessment Battery (NAB): Mazes
-
Verbal Learning: Hopkins Verbal Learning Test–Revised
Statistical analysis
IBM SPSS Statistics 21.0 was used to perform statistical analyses, p values are reported two-tailed and p < 0.05 was considered statistically significant. On demographic variables, groups were compared using independent sample t tests (for age and education), and a χ2 test (for gender). To test the cognitive performance differences, Analyses of covariance (ANCOVAs) were performed in which age, gender, and education level were included as covariates. For cortical thickness between-group comparisons, ANCOVA was used in which age and gender were included as covariates. Corrections for multiple comparisons were performed using the false discovery rate (FDR) with the Benjamini-Hochberg method50, and the significance level was set at 0.05.
To assess factors that explain variance in cognitive performance in the SZH group, a series of multiple regression models were constructed for each cognitive domain under study and the overall composite score. Duration of illness, gender, medication (olanzapine equivalent), education, PANSS positive, and PANSS negative were entered as independent variables and significance was defined as p < 0.05.
Partial correlations were performed for SZH and control group separately, with age and education as control variables. Follow-up analyses also included medication use as an additional covariate and are reported in Supplementary Tables 6 and 7. The ROIs encompassed a set of frontal and temporal regions where previous work has identified structure-cognition relationships in SZH that differ from those seen in controls. The frontal ROIs were: superior frontal (bilateral) and middle frontal (as shown by Hanford et al.29, measure: overall cognitive performance), inferior frontal (as shown by Oertel-Knöchel et al.35, measure: subjective cognitive dysfunction). Temporal ROIs were: transverse temporal (bilateral) (as shown by Hartberg et al.34, measure: processing speed), superior temporal (bilateral) (as shown by Ehrlich et al.36, measure: working memory; and Edgar et al.33, measure: attention) and middle temporal (as shown by Ehrlich et al.36, measure: working memory; and Hartberg et al.34, measure: processing speed). To control for multiple comparisons, FDR corrections were applied as above.
To test for differences in correlations between groups, a frequentist approach was taken using a percentile bootstrap method51. Between-group differences were assessed when a significant correlation was identified in one of the groups (FDR-corrected). Exploratory analyses also tested for between-group differences in correlations when a correlation was seen to be significant prior to FDR correction. Using a percentile bootstrap method with 5000 iterations, the distribution of bootstrap correlation differences is assessed to determine significance. A significant difference in correlations is inferred if the 95% percentile bootstrap confidence intervals do not overlap with zero. This analysis was performed in Matlab using code adapted from https://github.com/GRousselet/blog/tree/master/comp2dcorr and is more robust than other approaches87.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The dataset is freely available in the following website: http://fcon_1000.projects.nitrc.org/indi/retro/cobre.html.
Code availability
The MATLAB code that used for statistical analyses is freely available in the following website: https://github.com/GRousselet/blog/tree/master/comp2dcorr.
References
Rund, B. R. A review of longitudinal studies of cognitive functions in schizophrenia patients. Schizophrenia Bull. 24, 425–435 (1998).
Rund, B. R. The research evidence for schizophrenia as a neurodevelopmental disorder. Scand. J. Psychol. 59, 49–58 (2018).
DeLisi, L. E., Szulc, K. U., Bertisch, H. C., Majcher, M. & Brown, K. Understanding structural brain changes in schizophrenia. Dialogues Clin. Neurosci. 8, 71–78 (2006).
Gong, Q. et al. A neuroanatomical signature for schizophrenia across different ethnic groups. Schizophrenia Bull. 41, 1266–1275 (2015).
Knowles, E. E., David, A. S. & Reichenberg, A. Processing speed deficits in schizophrenia: reexamining the evidence. Am. J. psychiatry 167, 828–835 (2010).
Nuechterlein, K. H. et al. Attention/vigilance in schizophrenia: performance results from a large multi-site study of the Consortium on the Genetics of Schizophrenia (COGS). Schizophr. Res. 163, 38–46 (2015).
Forbes, N. F., Carrick, L. A., McIntosh, A. M. & Lawrie, S. M. Working memory in schizophrenia: a meta-analysis. Psychological Med. 39, 889–905 (2009).
Manglam, M. K. & Das, A. Verbal learning and memory and psychopathology in schizophrenia. Asian J. Psychiatry 6, 417–420 (2013).
Zhang, B. et al. Gender differences measured by the MATRICS consensus cognitive battery in chronic schizophrenia patients. Sci. Rep. 7, 11821 (2017).
Charernboon, T. & Patumanond, J. Social cognition in schizophrenia. Ment. Illn. 9, 7054 (2017).
Green, M. F. Cognitive impairment and functional outcome in schizophrenia and bipolar disorder. J. Clin. psychiatry 67, 36–42 (2006). Suppl 9, 3-8discussion.
Green, M. F., Kern, R. S., Braff, D. L. & Mintz, J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “Right Stuff”? Schizophrenia Bull. 26, 119–136 (2000).
Lepage, M., Bodnar, M. & Bowie, C. R. Neurocognition: clinical and functional outcomes in schizophrenia. Can. J. Psychiatry Rev. canadienne de. Psychiatr. 59, 5–12 (2014).
Tripathi, A., Kar, S. K. & Shukla, R. Cognitive deficits in schizophrenia: understanding the biological correlates and remediation strategies. Clin. Psychopharmacol. Neurosci. 16, 7–17 (2018).
Davies, G., Fowler, D. & Greenwood, K. Metacognition as a mediating variable between neurocognition and functional outcome in first episode psychosis. Schizophrenia Bull., sbw128. https://doi.org/10.1093/schbul/sbw128 (2016).
Best, M. W., Milanovic, M., Iftene, F. & Bowie, C. R. A randomized controlled trial of executive functioning training compared with perceptual training for schizophrenia spectrum disorders: effects on neurophysiology, neurocognition, and functioning. Am. J. Psychiatry 176, 297–306 (2019).
van Erp, T. G. M. et al. Cortical brain abnormalities in 4474 individuals with schizophrenia and 5098 control subjects via the enhancing neuro imaging genetics through meta analysis (ENIGMA) consortium. Biol. psychiatry 84, 644–654 (2018).
Alkan, E., Davies, G., Greenwood, K. & Evans, S. Brain structural correlates of functional capacity in first-episode psychosis. Sci. Rep. 10, 17229 (2020).
Wannan, C. M. J. et al. Evidence for network-based cortical thickness reductions in schizophrenia. Am. J. Psychiatry 176, 552–563 (2019).
Assunção Leme, I. B. et al. Is there an association between cortical thickness, age of onset, and duration of illness in schizophrenia? CNS Spectr. 18, 315–321 (2013).
Xiao, Y. et al. Altered cortical thickness related to clinical severity but not the untreated disease duration in schizophrenia. Schizophrenia Bull. 41, 201–210 (2015).
Godwin, D., Alpert, K. I., Wang, L. & Mamah, D. Regional cortical thinning in young adults with schizophrenia but not psychotic or non-psychotic bipolar I disorder. Int. J. Bipolar Disord. 6, 16 (2018).
Padmanabhan, J. L. et al. Correlations between brain structure and symptom dimensions of psychosis in schizophrenia, Schizoaffective, and psychotic bipolar I disorders. Schizophrenia Bull. 41, 154–162 (2014).
Carruthers, S. P., Van Rheenen, T. E., Gurvich, C., Sumner, P. J. & Rossell, S. L. Characterising the structure of cognitive heterogeneity in schizophrenia spectrum disorders. A systematic review and narrative synthesis. Neurosci. Biobehav. Rev. 107, 252–278 (2019).
Cobia, D. J., Csernansky, J. G. & Wang, L. Cortical thickness in neuropsychologically near-normal schizophrenia. Schizophr. Res. 133, 68–76 (2011).
Yasuda, Y. et al. Brain morphological and functional features in cognitive subgroups of schizophrenia. Psychiatry Clin. Neurosci. 74, 191–203 (2020).
Pan, Y. et al. Morphological profiling of schizophrenia: cluster analysis of MRI-Based cortical thickness data. Schizophrenia Bull. 46, 623–632 (2020).
Czepielewski, L. S., Wang, L., Gama, C. S. & Barch, D. M. The relationship of intellectual functioning and cognitive performance to brain structure in Schizophrenia. Schizophrenia Bull. 43, 355–364 (2017).
Hanford, L. C., Pinnock, F., Hall, G. B. & Heinrichs, R. W. Cortical thickness correlates of cognitive performance in cognitively-matched individuals with and without schizophrenia. Brain Cognition 132, 129–137 (2019).
Heinrichs, R. W. et al. Cortical thinning in network-associated regions in cognitively normal and below-normal range schizophrenia. Schizophr. Res Treat. 2017, 9760905 (2017).
Wang, L. et al. Progressive deformation of deep brain nuclei and hippocampal-amygdala formation in schizophrenia. Biol. psychiatry 64, 1060–1068 (2008).
Voineskos, A. N. et al. Diffusion tensor tractography findings in schizophrenia across the adult lifespan. Brain 133, 1494–1504 (2010).
Edgar, J. C. et al. Temporal and frontal cortical thickness associations with M100 auditory activity and attention in healthy controls and individuals with schizophrenia. Schizophr. Res 140, 250–257 (2012).
Hartberg, C. B. et al. Brain cortical thickness and surface area correlates of neurocognitive performance in patients with schizophrenia, bipolar disorder, and healthy adults. J. Int. Neuropsychological Soc. 17, 1080–1093 (2011).
Oertel-Knöchel, V. et al. Association between psychotic symptoms and cortical thickness reduction across the schizophrenia spectrum. Cereb. Cortex 23, 61–70 (2013).
Ehrlich, S. et al. Associations of cortical thickness and cognition in patients with schizophrenia and healthy controls. Schizophrenia Bull. 38, 1050–1062 (2011).
Calhoun, V. D. et al. Exploring the psychosis functional connectome: aberrant intrinsic networks in schizophrenia and bipolar disorder. Front. Psychiatry 2, 75–75 (2012).
Hanlon, F. M. et al. Bilateral hippocampal dysfunction in schizophrenia. NeuroImage 58, 1158–1168 (2011).
Mayer, A. R. et al. Functional imaging of the hemodynamic sensory gating response in schizophrenia. Hum. Brain Mapp. 34, 2302–2312 (2013).
Stephen, J. M. et al. Using joint ICA to link function and structure using MEG and DTI in schizophrenia. NeuroImage 83, 418–430 (2013).
Kay, S. R., Fiszbein, A. & Opler, L. A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia Bull. 13, 261–276 (1987).
Cabral, C. et al. Classifying schizophrenia using multimodal multivariate pattern recognition analysis: evaluating the impact of individual clinical profiles on the neurodiagnostic performance. Schizophrenia Bull. 42, S110–S117 (2016).
Aine, C. J. et al. Multimodal neuroimaging in schizophrenia: description and dissemination. Neuroinformatics 15, 343–364 (2017).
Çetin, M. S. et al. Thalamus and posterior temporal lobe show greater inter-network connectivity at rest and across sensory paradigms in schizophrenia. NeuroImage 97, 117–126 (2014).
Destrieux, C., Fischl, B., Dale, A. & Halgren, E. A sulcal depth-based anatomical parcellation of the cerebral cortex. Neuroimage 47, https://doi.org/10.1016/S1053-8119(09)71561-7 (2009).
Green, M. F. et al. Approaching a consensus cognitive battery for clinical trials in schizophrenia: The NIMH-MATRICS conference to select cognitive domains and test criteria. Biol. Psychiatry 56, 301–307 (2004).
Nuechterlein, K. H. et al. The MATRICS consensus cognitive battery, part 1: test selection, reliability, and validity. Am. J. psychiatry 165, 203–213 (2008).
Marder, S. R. & Fenton, W. Measurement and treatment research to improve cognition in schizophrenia: NIMH MATRICS initiative to support the development of agents for improving cognition in schizophrenia. Schizophr. Res 72, 5–9 (2004).
Robert S. Kern, P. D. et al. The MATRICS consensus cognitive battery, part 2: co-norming and standardization. Am. J. Psychiatry 165, 214–220 (2008).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B (Methodol.) 57, 289–300 (1995).
Wilcox, R. R. Comparing dependent robust correlations. Br. J. Math. Stat. Psychol. 69, 215–224 (2016).
Husa, A. P. et al. Lifetime antipsychotic medication and cognitive performance in schizophrenia at age 43 years in a general population birth cohort. Psychiatry Res. 247, 130–138 (2017).
Goldman, A. L. et al. Widespread reductions of cortical thickness in schizophrenia and spectrum disorders and evidence of heritability. Arch. Gen. Psychiatry 66, 467–477 (2009).
Takayanagi, Y. et al. Volume reduction and altered sulco-gyral pattern of the orbitofrontal cortex in first-episode schizophrenia. Schizophr. Res 121, 55–65 (2010).
Parent, A. & Carpenter, M. B. Carpenter’s human neuroanatomy. (Williams & Wilkins, 1996).
Rimol, L. M. et al. Cortical volume, surface area, and thickness in schizophrenia and bipolar disorder. Biol. psychiatry 71, 552–560 (2012).
Harrison, P. J. The neuropathology of schizophrenia: a critical review of the data and their interpretation. Brain 122, 593–624 (1999).
Thune, J. J., Uylings, H. B. M. & Pakkenberg, B. No deficit in total number of neurons in the prefrontal cortex in schizophrenics. J. Psychiatr. Res. 35, 15–21 (2001).
Suga, M. et al. Reduced gray matter volume of Brodmann’s Area 45 is associated with severe psychotic symptoms in patients with schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 260, 465–473 (2010).
Walton, E. et al. Positive symptoms associate with cortical thinning in the superior temporal gyrus via the ENIGMA schizophrenia consortium. Acta Psychiatr. Scand. 135, 439–447 (2017).
Walton, E. et al. Prefrontal cortical thinning links to negative symptoms in schizophrenia via the ENIGMA consortium. Psychological Med. 48, 82–94 (2018).
Williams, M. R. et al. Changes in cortical thickness in the frontal lobes in schizophrenia are a result of thinning of pyramidal cell layers. Eur. Arch. Psychiatry Clin. Neurosci. 263, 25–39 (2013).
Andreasen, N. C. et al. Progressive brain change in schizophrenia: a prospective longitudinal study of first-episode schizophrenia. Biol. psychiatry 70, 672–679 (2011).
Ding, Y. et al. Enhanced global-brain functional connectivity in the left superior frontal gyrus as a possible endophenotype for schizophrenia. Front Neurosci. 13, 145 (2019).
Oertel, V. et al. Reduced laterality as a trait marker of schizophrenia-evidence from structural and functional neuroimaging. J. Neurosci. 30, 2289–2299 (2010).
Quintana, J. et al. Prefrontal-posterior parietal networks in schizophrenia: primary dysfunctions and secondary compensations. Biol. Psychiatry 53, 12–24 (2003).
Gaser, C., Nenadic, I., Volz, H.-P., Büchel, C. & Sauer, H. Neuroanatomy of ‘Hearing Voices’: a frontotemporal brain structural abnormality associated with auditory hallucinations in schizophrenia. Cereb. Cortex 14, 91–96 (2004).
Modinos, G. et al. Neuroanatomy of auditory verbal hallucinations in schizophrenia: a quantitative meta-analysis of voxel-based morphometry studies. Cortex 49, 1046–1055 (2013).
Chen, C. et al. Impaired processing speed and attention in first-episode drug naive schizophrenia with deficit syndrome. Schizophr. Res. 159, 478–484 (2014).
Nestor, P. G. et al. Dissociable contributions of MRI volume reductions of superior temporal and fusiform gyri to symptoms and neuropsychology in schizophrenia. Schizophr. Res. 91, 103–106 (2007).
Brandt, C. L. et al. Assessing brain structural associations with working-memory related brain patterns in schizophrenia and healthy controls using linked independent component analysis. NeuroImage. Clin. 9, 253–263 (2015).
Hartberg, C. B. et al. Investigating relationships between cortical thickness and cognitive performance in patients with schizophrenia and healthy adults. Psychiatry Res. 182, 123–133 (2010).
Holleran, L. et al. The relationship between white matter microstructure and general cognitive ability in patients with schizophrenia and healthy participants in the ENIGMA consortium. Am. J. Psychiatry 177, 537–547 (2020).
Davies, G. et al. Study of 300,486 individuals identifies 148 independent genetic loci influencing general cognitive function. Nat. Commun. 9, 2098 (2018).
Harvey, P. D. et al. Genome-wide association study of cognitive performance in U.S. veterans with schizophrenia or bipolar disorder. Am. J. Med Genet Part B 183, 181–194 (2020).
Lee, J. J. et al. Gene discovery and polygenic prediction from a genome-wide association study of educational attainment in 1.1 million individuals. Nat. Genet. 50, 1112–1121 (2018).
Savage, J. E. et al. Genome-wide association meta-analysis in 269,867 individuals identifies new genetic and functional links to intelligence. Nat. Genet. 50, 912–919 (2018).
Templ, M., Gussenbauer, J. & Filzmoser, P. Evaluation of robust outlier detection methods for zero-inflated complex data. J. Appl. Stat. 47, 1144–1167 (2020).
Walfish, S. A review of statistical outlier methods. J. Pharm. Technol. 30, 82–86 (2006).
Rousseeuw, P. J. & Croux, C. Alternatives to the median absolute deviation. J. Am. Stat. Assoc. 88, 1273–1283 (1993).
Seo, S. A Review and Comparison of Methods for Detecting Outliers in Univariate Data Sets Master of Science thesis, Kyunghee University, (2006).
First, M. B. & Gibbon, M. in Comprehensive handbook of psychological assessment, Vol. 2: Personality assessment. 134-143 (John Wiley & Sons Inc, 2004).
Dale, A. M., Fischl, B. & Sereno, M. I. Cortical surface-based analysis: i. segmentation and surface reconstruction. NeuroImage 9, 179–194 (1999).
Fischl, B. et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 33, 341–355 (2002).
Fischl, B. FreeSurfer. NeuroImage 62, 774–781 (2012).
Fischl, B., Sereno, M. I. & Dale, A. M. Cortical surface-based analysis: ii: inflation, flattening, and a surface-based coordinate system. NeuroImage 9, 195–207 (1999).
Wilcox, R. R. & Rousselet, G. A. A guide to robust statistical methods in neuroscience. Curr. Protoc. Neurosci. 82, 8.42.41–48.42.30 (2018).
Acknowledgements
Data was downloaded from the Collaborative Informatics and Neuroimaging Suite Data Exchange tool (COINS) and data collection was performed at the Mind Research Network, and funded by a Center of Biomedical Research Excellence (COBRE) grant 5P20RR021938/P20GM103472 from the NIH to Dr. Vince Calhoun. We thank The Center for Biomedical Research Excellence (COBRE) for publicly releasing the dataset.
Author information
Authors and Affiliations
Contributions
E.A.: Writing- Original draft preparation, Formal analysis, Methodology, Conceptualization G.D.: Writing- Original draft preparation, Resources, Data Curation, Writing - Review & Editing S.E.: Writing- Original draft preparation, Formal analysis, Methodology, Conceptualization.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Alkan, E., Davies, G. & Evans, S.L. Cognitive impairment in schizophrenia: relationships with cortical thickness in fronto-temporal regions, and dissociability from symptom severity. npj Schizophr 7, 20 (2021). https://doi.org/10.1038/s41537-021-00149-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41537-021-00149-0