Abstract
There is limited information about the initiation of triple therapy (TT) in patients with chronic obstructive pulmonary disease (COPD) in primary care. This was an observational, population-based study in patients identified from a primary care electronic medical records database in Catalonia from 2011 to 2015 aimed to identify the use of TT in patients with newly diagnosed COPD. A total of 69,668 newly diagnosed patients were identified of whom 11,524 (16.5%) initiated TT, of whom 8626 initiated TT at or immediately after COPD diagnosis. Among them, 72.3% were GOLD A/B, 14.6% were frequent exacerbators, and 7.1% had asthma–COPD overlap (ACO). Variables associated with TT initiation were: male sex, older age, previous exacerbations, ACO, a previous treatment regimen containing an inhaled corticosteroid, previous pneumonia, and history of lung cancer. A significant number of COPD patients in Primary Care initiated TT shortly after or even before an established COPD diagnosis.
Similar content being viewed by others
Introduction
Treatments administered for patients with chronic obstructive pulmonary disease (COPD) in primary care do not always follow national or international guidelines1,2,3,4. Patients with milder forms of the disease are frequently overtreated and, although less frequently, patients with more severe COPD may be undertreated1,2,3,4. In a majority of countries, the initial point of contact for patients with respiratory symptoms is through primary care where targeted detection programs for the early diagnosis of COPD are supported5,6.
The availability of spirometry in primary care is key for early and accurate diagnosis of COPD7, and initial treatment should be implemented according to guidelines. The Global Strategy for Obstructive Lung Disease (GOLD) recommends classifying patients according to severity of symptoms and history of exacerbations into four categories. Initial treatment is based on one or two long-acting bronchodilators, or the combination of a long-acting beta-2 agonist (LABA) and an inhaled corticosteroid (ICS) for more symptomatic patients with frequent exacerbations8. When treatment with long-acting bronchodilators or with LABA/ICS is not enough to control symptoms or prevent exacerbations, treatment may escalate to triple therapy (TT) with two bronchodilators (a LABA and a long-acting antimuscarinic agent [LAMA]) and ICS. Similarly, the Spanish guidelines also recommend TT as a step up from dual bronchodilation (LABA/LAMA) or from LABA/ICS, but not as initial therapy9.
Studies from different countries have demonstrated a frequent use of TT in patients considered at low risk (patients with GOLD A or B disease). A recent study in the United Kingdom showed that 13.7% of GOLD A and 26.2% of GOLD B received TT2; similarly, data from Switzerland observed a use of TT in 13.8% of GOLD A and 28.2% of GOLD B patients1. It is important to understand whether this represents overtreatment of COPD or whether some of these patients could have been GOLD D patients that improved in the GOLD classification because of the efficacy of TT. In order to understand the reason for TT prescription in primary care, it is important to analyze prescription patterns in large administrative databases. The current study was conducted with the aim of characterizing newly diagnosed patients with COPD initiating TT in primary care and investigating the factors associated with TT prescription in these incident cases with COPD.
Results
Population of the study
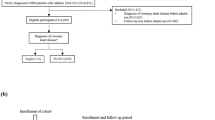
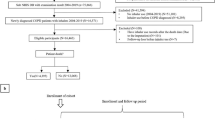
A total of 69,668 newly diagnosed (incident) COPD patients were identified; of whom 11,524 (16.5%) initiated TT at some point during the study period. Among these patients, 2898 (25.2%) initiated TT prior to COPD diagnosis, and 8626 (74.8%) at or after COPD diagnosis (Fig. 1).
Clinical characteristics of patients initiating and not initiating TT, and subgroups according to TT period of initiation
The majority of incident patients with COPD were men (68.6%), with mean age of 68.3 years (standard deviation [SD]: 12.3 years). Less than half (44.3%) had spirometry tests performed within 12 months before and 2 weeks after the index date, mean FEV1 (% predicted) was 65.3% (SD: 19.6%) and 47.6% had at least one exacerbation in the previous year (Table 1).
Incident patients with COPD who initiated TT more frequently had previous exacerbations (51.4 % versus 46.9%; χ2 test p < 0.001) and had more previous visits to primary care (9.8 [SD: 7.4] versus 8.1 [SD: 6.3]; T-test p < 0.001); but less frequently had available spirometry records (34.5% versus 46.3%; χ2 test p < 0.001) than patients who did not initiate TT. These differences were also observed between patients who initiated TT before diagnosis, with more exacerbations (57.2% versus 49.4%; χ2 test p < 0.001) and patients with spirometry tests (32.1% versus 35.3%; χ2 test p < 0.001) compared to patients who initiated TT at or after COPD diagnosis. Generally, patients who initiated TT prior to diagnosis also appeared to have a higher prevalence of comorbidities (particularly respiratory related) than patients who initiated TT at or after COPD diagnosis. No significant differences were observed in blood eosinophil concentration (BEC) between groups (Table 1).
Characteristics of patients who initiated TT at or after COPD diagnosis and according to severity of disease
Among the 8626 patients who initiated TT at or after COPD diagnosis, 6237 (72.3%) were GOLD A/B and 1260 (14.6%) were frequent exacerbators. A total of 615 (7.1%) were classified as asthma–COPD overlap (ACO) (Table 2). ACO patients had a lower proportion of males (53.2%), were slightly younger 65.7 years (SD: 11.9 years), and had a higher BEC (275 cells/ml; SD: 185) than patients who did not have ACO.
Regarding spirometry, it was more frequently performed in GOLD C/D patients with up to 61.1% compared to 25.5% of GOLD A/B, and these patients also had the lowest FEV1 (%) values (40.7%, SD: 18.5) compared to GOLD A/B (66.7%, SD: 11.7). Distribution of comorbidities was similar between subgroups (Table 2).
Patients with ACO were the group most frequently treated with a regimen including an ICS before initiating TT (54.8%) compared with the 41.2% for all the groups. About a quarter of patients had no previous treatment the year before initiating TT (Table 2).
Time between COPD diagnosis and initiation of triple therapy
Over the study period, median time between diagnosis and TT initiation was 49 days (interquartile range [IQR]: −1; 439 days) accounting for all patients who initiated TT at some point during the study period, before or after COPD diagnosis. Restricting the sample to patients who initiated TT at or after COPD diagnosis, median time from COPD diagnosis to TT initiation was 196 days (IQR: 15; 620 days). The median time to TT was shorter for GOLD C/D patients (151 days; IQR: 11; 535 days) compared to GOLD A/B (217 days; IQR: 18; 644 days) (Fig. 2). Regarding phenotypes, time to TT initiation was shorter in frequent exacerbators (123 days; IQR: 13; 462 days) compared to infrequent (160 days; IQR: 10; 560 days) and non-exacerbators (254 days; IQR: 122; 686 days) (Table 3 and Fig. 3).
Factors independently associated with time to TT initiation
Patients with prior treatment including an ICS showed a higher likelihood to initiate TT earlier than patients with no prior ICS treatment (Table 4). Similarly, more severe GOLD stage (C/D) (HR = 2.36, 95% CI: 2.21–2.53; logistic regression analysis p < 0.001), followed by ACO (HR = 1.89, 95% CI: 1.74–2.06; p < 0.001), and exacerbators (HR = 1.56, 95% CI: 1.52–1.61; p < 0.001) were associated with earlier initiation of TT. Older age, male sex, and pneumonia and lung cancer were also significantly associated with higher probability of early TT initiation (Table 4).
Discussion
The results of the current study have shown that 16.5% of patients with a new diagnosis of COPD in primary care initiated TT during the 5-year study period. Interestingly one quarter of patients initiated TT even before the diagnosis of COPD was recorded in the electronic medical records. Patients who were frequent exacerbators or had ACO were more likely to initiate TT earlier, which is in accordance with COPD treatment recommendations8,9. However, it appears that BEC did not influence the decision to initiate TT in this study.
In order to analyze the characteristics of patients initiating TT and time to TT initiation we focused on the patients who initiated TT at or after diagnosis of COPD. The rationale of this was to avoid any bias that may be introduced by analyzing characteristics of patients who, at the time of prescribing, might have been diagnosed with other diseases, such as asthma, or not diagnosed at all. Moreover, the group of patients initiating TT at or after diagnosis of COPD constituted three quarters of the whole population.
The observed 16.5% of incident patients with COPD initiating TT represents an increase from the 12% observed in a previous study in Spain conducted between 2007 and 201210 and is higher than the 7.5% of patients initiating TT after COPD diagnosis observed in a large database study in the United States11. However, it is lower than the percentages observed in other European cohorts: Vetrano et al.12 found that 21% of patients with COPD had TT as their first inhaled prescription. In the United Kingdom, Wurst et al.13 observed that 23% of patients with incident COPD initiated TT, and another recent study described an increase in the use of TT as initial therapy from <1% in 2002 to 28% in 2014, with a small reduction to 22% in 201614. In Italy, a 6.3% from a population of +32,000 patients with COPD in primary care were treated with TT during the first year after diagnosis15. It is important to contrast these figures with the current GOLD recommendations that do not include TT as initial therapy in any of the GOLD groups, but only as step up when initial therapy is not effective in controlling symptoms or preventing exacerbations8,16,17.
After excluding patients who initiated TT before COPD diagnosis, the remaining incident patients with COPD initiated TT a median of 196 days after diagnosis, with a shorter period observed in frequent exacerbators and GOLD C/D patients, which is in line with treatment recommendations8. It is of note that around one quarter of newly diagnosed COPD patients did not have any inhaled treatment before TT. Among the remaining patients, the most frequent treatment received before TT was LABA/ICS followed by LABA/LAMA. These results are different from those observed in a previous study by Monteagudo et al.18 in over 34,000 prevalent COPD cases with TT from the same database, in whom the most frequent previous treatment was LAMA (22.5%) followed by LABA/ICS (15.2%). Mapel et al.19 in the United States also found that the most frequent treatment before TT was LAMA (15.4%) followed by LABA/ICS (7.7%). It might be that incident COPD cases initiating TT may be more symptomatic and, therefore, they are more frequently started already on LABA/ICS (and early stepped up to TT) instead of starting on monotherapies. It is of note that only 34.5% of patients who initiated TT had spirometry recorded in their clinical records. The underuse of spirometry is still a pending issue in primary care that may affect diagnostic accuracy.
In multivariate analysis, we observed that some variables that define the severity of the disease were significantly associated with the initiation of TT in newly diagnosed patients with COPD. This expected finding included characteristics such as increasing age, more severe GOLD stage, and more frequent exacerbations. Another characteristic significantly associated with TT initiation was the ACO phenotype, which is also in alignment with current recommendations, that recommend the use of ICS in combination with long-acting bronchodilators to prevent exacerbations, in particular in patients with eosinophilic profile or a previous diagnosis of asthma8,9.
However, other identified factors associated with the initiation of TT were unexpected. Male sex was significantly associated with TT initiation in patients with incident COPD. This gender bias is difficult to explain and is not linked to the well-described diagnostic bias20, since all patients had a diagnosis of COPD. This finding should be analyzed in other studies that investigate whether it is due to a trend toward overprescription in men, or, alternatively an underprescription in women.
Another surprising finding is the significant association of TT initiation and the diagnosis of pneumonia. It is well recognized that the use of ICS is a risk factor for pneumonia in patients with COPD21 and, therefore, the opposite association would be expected. A hint to explain this paradox can be found in the next characteristic associated with initiation of TT, which is the diagnosis of lung cancer. The possible explanation could be that both patients with episodes of pneumonia and lung cancer may visit the primary care physician more frequently with more respiratory complaints, therefore, stepping up treatment to include ICS may be more likely. However, the association between the initiation of TT and pneumonia suggests that PCP may not be aware of the risk of pneumonia associated with the use of ICS, in particular in patients with more severe disease, underweight, and with low BEC22,23.
Finally, we did not find an association between TT initiation and BEC. Whilst it must be considered that the relevance of the use of BEC to guide ICS treatment in COPD has only appeared in recent years, the 2012 edition of the Spanish Guideline on treatment of COPD already mentioned the use of BEC to classify patients as ACO and personalize the prescription of ICS predominantly to patients with this phenotype24. Our data and other large database studies18,25 show that BEC are available for the great majority of patients with COPD in primary care and therefore can be a useful biomarker for the appropriate use of ICS in COPD26.
In conclusion, we have observed that around 15% of newly diagnosed patients with COPD initiate TT. Among them, one quarter had no previous inhaled therapy. Although these findings may not be consistent with current COPD guidelines, the observation that patients with ACO and frequent exacerbations were more likely to start TT and started TT earlier is in alignment with treatment recommendations.
Methods
Study design
This was a retrospective study with longitudinal follow-up. Data analyzed were obtained from the Information System for the Development of Research in Primary Care (SIDIAP) database; this database includes anonymized electronic medical records from 270 primary care centers with a reference population of 5.8 million people in Catalonia (Spain), which represents more than 80% of the total population27. This database has been used and validated for epidemiological research in respiratory diseases25,28.
Patient selection
Included patients were those over 40 years of age with a first diagnosis of COPD (International Classification of Disease—10th Edition) between January 1, 2011 and December 31, 2015. Patients were required to have at least 12 months of available medical record data prior to diagnosis of COPD. Among incident patients with COPD, those who initiated TT were defined as having prescriptions for LAMA, LABA, and ICS with an overlap of 30 days or more. If a patient had a gap of 60 days for prescription of one or more of the three compounds, they were considered to no longer be persistent on TT.
Patient subgroups
According to the time of initiation of TT, patients were classified into those who initiated TT before the date of COPD diagnosis and those who initiated TT at or after the date of diagnosis.
The phenotypes of the patients were also identified9. Those with a concomitant diagnosis of asthma recorded I clinical records were included in the ACO phenotype29,30. Patients with two or more exacerbations during the year before initiation of TT were classified as a frequent exacerbator phenotype, patients with only one exacerbation were classified as infrequent exacerbators, and the remaining COPD patients were defined as non-exacerbators. ACO and the exacerbator phenotypes were not mutually exclusive.
Patients were also classified according to the GOLD 2013 categories based on spirometry and exacerbation data as low risk (GOLD A/B) and high risk (GOLD C/D)31.
Study measurements
Detailed information at the time of diagnosis was collected on the following variables: age, sex, comorbidities, smoking history, history of exacerbations, and treatments received during the year before the diagnosis of COPD. Results of spirometry and blood analysis with BEC at diagnosis were also collected.
Exacerbation episodes were identified according to previously published algorithms10,28. Briefly, we used a diagnostic code indicative of a respiratory exacerbation, or receipt of corticosteroids and/or antibiotics used for treating the episode. Exacerbations leading to hospitalizations or treated as part of inpatient care were not available in the data. To avoid misclassification and over-estimation of exacerbations, consecutive episodes with less than 21 days between prescriptions and physician visits were considered as a single event.
The study was approved by the Research and Ethics Committee of the IDIAP Jordi Gol Institute of Research in Primary Care (Barcelona, Spain). Since anonymized data were collected retrospectively, no informed consent was considered necessary.
Statistical analysis
Description of variables was performed with absolute frequencies and corresponding percentages. Continuous variables were described using the mean and SD, while time from diagnosis to TT initiation in days was described using the median and IQR.
Categorical variables were compared using the χ2 or Fisher exact test where applicable. Quantitative variables were compared using the T-test or Mann–Whitney U test.
The variables significantly and independently associated with time to initiation of TT in newly diagnosed patients were identified by means of a logistic regression analysis. The results were described using hazard ratios with a 95% confidence interval and p values. All statistical analyses were performed using the statistical software package Statistical Package for Social Science (SPSS) version 20.0 (IBM, Chicago, IL, USA). A p value <0.05 was considered significant.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The data that support the findings of this study belong to the Catalan Healthcare System and are not publicly available. They were used under license for the current study. Data are, however, available from the corresponding authors upon reasonable request and with permission of Information System for the Development of Research in Primary Care (SIDIAP).
References
Grewe, F. A. et al. Compliance of pharmacotherapy with GOLD guidelines: a longitudinal study in patients with COPD. Int J. Chron. Obstruct Pulmon Dis. 15, 627–635 (2020).
Halpin, D. M. G., de Jong, H. J. I., Carter, V., Skinner, D. & Price, D. Distribution, temporal stability and appropriateness of therapy of patients with COPD in the UK in relation to GOLD 2019. EClinicalMedicine 14, 32–41 (2019).
Izquierdo, J. L. et al. Characteristics of COPD patients managed in respiratory medicine departments in Spain, according to GOLD groups and GesEPOC clinical phenotypes. Arch. Bronconeumol. 54, 559–567 (2018).
Izquierdo, J. L. et al. Clinical management of COPD in a real-world setting. A big data analysis. Arch. Bronconeumol. 57, 94–100 (2021).
Llordés, M. et al. Which is the best screening strategy for COPD among smokers in Primary Care? COPD 14, 43–51 (2017).
Soriano, J. B., Paris, Molina, Miravitlles, J. & Combining, M. Case-finding methods for COPD in primary care: a large, two-stage design study. Int J. Tub. Lung Dis. 22, 106–111 (2018).
Monteagudo, M. et al. Variability in the performing of spirometry and its consequences in the treatment of COPD in primary care. Arch. Bronconeumol. 47, 226–233 (2011).
Singh, D. et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: the GOLD science committee report 2019. Eur. Respir. J. 53, 1900164 (2019).
Miravitlles, M. et al. Spanish guidelines for management of chronic obstructive pulmonary disease (GesEPOC) 2017. Pharmacological treatment of stable phase. Arch. Bronconeumol. 53, 324–335 (2017).
Barrecheguren, M. et al. Treatment patterns in COPD patients newly diagnosed in primary care. A population-based study. Respir. Med. 11, 47–53 (2016).
Simeone, J. C. et al. Initiation of triple therapy maintenance treatment among patients with COPD in the US. Int J. Chron. Obstruct Pulmon Dis. 12, 73–83 (2017).
Vetrano, D. L. et al. Triple inhaled therapy in COPD patients: determinants of prescription in primary care. Respir. Med. 154, 12–17 (2019).
Wurst, K. E., Shukla, A., Muellerova, H. & Davis, K. J. Respiratory pharmacotherapy use in patients newly diagnosed with chronic obstructive pulmonary disease in a primary care setting in the UK: a retrospective cohort study. COPD 11, 521–530 (2014).
Bloom, C. I., Elkin, S. L. & Quint, J. K. Changes in COPD inhaler prescriptions in the United Kingdom, 2000 to 2016. Int J. Chron. Obstruct Pulmon Dis. 14, 279–287 (2019).
Di Marco, F. et al. Characteristics of newly diagnosed COPD patients treated with triple inhaled therapy by general practitioners: a real world Italian study. NPJ Prim. Care Respir. Med. 27, 51 (2017).
Calverley, P. M. A., Magnussen, H., Miravitlles, M. & Wedzicha, J. A. Triple therapy in COPD: what we know and what we don’t. COPD 14, 648–662 (2017).
López-Campos, J. L. et al. The clinical implications of triple therapy in fixed-dose combination in COPD: from the trial to the patient. Arch. Bronconeumol. 56, 242–248 (2020).
Monteagudo, M. et al. Treatment pathways before and after triple therapy in COPD: a population-based study in Primary Care in Spain. Arch. Bronconeumol. 57, 205–213 (2020).
Mapel, D. et al. A retrospective study to assess clinical characteristics and time to initiation of open-triple therapy among patients with chronic obstructive pulmonary disease, newly established on long-acting mono or combination therapy. Int J. Chron. Obstruct Pulmon Dis. 12, 1825–1836 (2017).
Miravitlles, M. et al. Attitudes towards the diagnosis of chronic obstructive pulmonary disease in Primary Care. Arch. Bronconeumol. 42, 3–8 (2006).
Kew, K. M. & Seniukovich, A. Inhaled steroids and risk of pneumonia for chronic obstructive pulmonary disease. Cochrane Systematic Review. https://doi.org/10.1002/14651858.CD010115.pub2 (2014).
Crim, C. et al. Pneumonia risk with inhaled fluticasone furoate and vilanterol compared with vilanterol alone in patients with COPD. Ann. Am. Thorac. Soc. 12, 27–34 (2015).
Pavord, I. D., Lettis, S., Anzueto, A. & Barnes, N. Blood eosinophil count and pneumonia risk in patients with chronic obstructive pulmonary disease: a patient-level meta-analysis. Lancet Respir. Med. 4, 731–741 (2016).
Miravitlles, M. et al. Spanish COPD guidelines (GesEPOC). Pharmacological treatment of stable COPD. Arch. Bronconeumol. 48, 247–257 (2012).
Miravitlles, M., Monteagudo, M., Solntseva, I. & Alcázar, B. Blood eosinophil counts and their variability and risk of exacerbations in COPD: a population-based study. Arch. Bronconeumol. 57, 13–20 (2021).
Kolsum, U., Southworth, T., Jackson, N. & Singh, D. Blood eosinophil counts in COPD patients compared to controls. Eur. Respir. J. 54, 1900633 (2019).
Bolíbar, B. et al. SIDIAP database: electronic clinical records in primary care as a source of information for epidemiologic research. Med Clin. 138, 617–621 (2012).
Monteagudo, M., Roset, M., Rodríguez-Blanco, T., Muñoz, L. & Miravitlles, M. Characteristics of COPD patients initiating treatment with aclidinium or tiotropium in primary care in Catalonia: a population-based study. Int J. Chron. Obstruct Pulmon Dis. 12, 1145–1152 (2017).
Nuñez, A. et al. Practical guide to the identification and diagnosis of asthma-COPD overlap (ACO). COPD 16, 1–7 (2019).
Soler-Cataluña, J. J. et al. Clinical characteristics and risk of exacerbations associated with different diagnostic criteria of asthma-COPD overlap. Arch. Bronconeumol. 56, 282–290 (2020).
Vestbo, J. et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am. J. Respir. Crit. Care Med. 187, 347–365 (2013).
Acknowledgements
The current study has been funded by an unrestricted grant from AstraZeneca that was also involved in study conception and protocol development. A.N. is the recipient of a Rio Hortega contract in the 2019 Strategic Action Health Call from the Instituto de Salud Carlos III for the years 2020–2022.
Author information
Authors and Affiliations
Contributions
D.L., N.D., and A.B. conceived and designed the study. M. Monteagudo and I.S. coordinated statistical analyses. M. Miravitlles and M. Monteagudo wrote manuscript. All authors contributed to study design and analysis, drafting, reviewing, and critically revising the paper, gave final approval of the version to be published, and agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
M. Miravitlles has received speaker fees from AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, Menarini, Rovi, Bial, Sandoz, Zambon, CSL Behring, Grifols, and Novartis; consulting fees from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Bial, Gebro Pharma, Kamada, CSL Behring, Laboratorios Esteve, Ferrer, Mereo Biopharma, Verona Pharma, TEVA, pH Pharma, Novartis, Sanofi, and Grifols; and research grants from GlaxoSmithKline and Grifols. M.B. has received speaker fees from Grifols, Menarini, CSL Behring, and GSK and consulting fees from GSK and Novartis. D.L., N.D., and A.B. are employees of Evidera. The authors confirm that there are no other conflicts of interest to report.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Monteagudo, M., Barrecheguren, M., Solntseva, I. et al. Clinical characteristics and factors associated with triple therapy use in newly diagnosed patients with COPD. npj Prim. Care Respir. Med. 31, 16 (2021). https://doi.org/10.1038/s41533-021-00227-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41533-021-00227-x
This article is cited by
-
Characteristics and treatment patterns of patients with asthma on multiple-inhaler triple therapy in Spain
npj Primary Care Respiratory Medicine (2022)