Abstract
Neuroinflammation plays a crucial role in the pathogenesis of Parkinson’s disease (PD), but controversies persist. Studies reporting concentrations of blood or cerebrospinal fluid (CSF) markers for patients with PD and controls were included and extracted. Pooled Hedges’g was adopted to illustrate comparisons, and covariates were used to explore sources of heterogeneity. Finally, 152 studies were included. Increased IL-6, TNF-α, IL-1β, STNFR1, CRP, CCL2, CX3CL1, and CXCL12 levels and decreased INF-γ and IL-4 levels were noted in the PD group. In addition, increased CSF levels of IL-6, TNF-α, IL-1β, CRP and CCL2 were revealed in patients with PD compared to controls. Consequently, significantly altered levels of inflammatory markers were verified between PD group and control, suggesting that PD is accompanied by inflammatory responses in both the peripheral blood and CSF. This study was registered with PROSPERO, CRD42022349182.
Similar content being viewed by others
Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative diseases, which exhibits diverse clinical features including motor and nonmotor symptoms1, and leads to decreased quality of daily life, disability or eventually death in the elderly2. PD is characterized by the selective loss of dopaminergic neurons in the substantia nigra (SN) pars compacta, but the exact aetiology remains unclear3. Increasing evidence has suggested that central and peripheral inflammation play vital roles in the pathologic features and symptoms of PD4, and several peripheral biomarkers exhibit tracing and detection accuracy for disease severity and progression4,5.
Varieties inflammatory markers, including cytokines such as the interleukin (IL) and tumour necrosis factor (TNF); chemokines such as chemokine ligand (CCL) and CX3 chemokine ligand (CX3CL); and the acute phase reactant protein C-reactive protein (CRP), have been reported as critical signalling molecules of immune activation that exert effects in the central nervous system (CNS) and periphery6. In addition, peripheral inflammation can contribute to the aetiology and progress of PD7. The less invasive markers present in peripheral blood and cerebrospinal fluid (CSF) can assist in better understanding the aetiology of PD and provide candidate biomarkers for the disease; however, their performances varies greatly in different studies due to differences in research sites and tools.
Previous reviews and meta-analyses have demonstrated that the levels of inflammatory markers in the peripheral blood and CSF of patients with PD differ from those for healthy populations8,9,10. However, some of these markers lack quantitative analyses, recent updated information, or comprehensive included inflammatory markers. To explore the real altered levels of each marker, this meta-analysis and systematic review aimed to verify whether the concentrations of specific inflammatory markers in peripheral blood and CSF differ quantitatively between patients with PD and normal controls.
Results
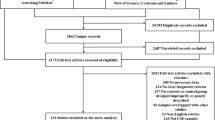
A total of 16,156 records were identified after literature searching, selection and deduplication, and 152 studies measuring peripheral blood or CSF inflammatory markers were finally included in the systematic reviews and meta-analyses (Fig. 1). The characteristics and quality assessments are listed in Supplementary Table 1–2 which encompassed 9,032 patients diagnosed with PD and 12,628 controls. In total, 92 markers were analysed, and the official marker names are presented in Supplementary Table 3. Performances and heterogeneity analyses of individual markers are shown in Supplementary Tables 4-5.
Comparisons of peripheral blood biomarkers between PD patients and control
Random-effects results demonstrated that patients with PD had higher peripheral blood levels of IL-6 (Hedges’ g 0.603; 95%CI 0.325 to 0.881, P < 0.001), TNF-α (Hedges’ g 0.593; 95%CI 0.293 to 0.894, P < 0.001), IL-1β (Hedges’ g 1.300; 95%CI 0.709 to 1.892, P < 0.001), soluble TNF receptor 1 (sTNFR1; Hedges’ g 0.449; 95%CI 0.004 to 0.894, P = 0.048), CRP (Hedges’ g 0.510; 95%CI 0.313 to 0.706, P < 0.001), CCL2 (Hedges’ g 0.911; 95%CI 0.246 to 1.576, P = 0.007), CX3CL1 (Hedges’ g 0.361; 95%CI 0.166 to 0.556, P < 0.001), CX chemokine ligand 12 (CXCL12; Hedges’ g 2.933; 95%CI 0.883 to 4.983, P = 0.005), insulin-like growth factor-1 (IGF-1; Hedges’ g 0.534; 95%CI 0.355 to 0.714, P < 0.001) and N-terminal pro-B-type natriuretic peptide (NT-pro BNP; Hedges’ g 0.533; 95%CI 0.256 to 0.809, P < 0.001). Furthermore, significantly decreasing concentrations were revealed for IFN-γ (Hedges’ g -0.385; 95%CI -0.743 to -0.026, P = 0.035), IL-4 (Hedges’ g -0.710; 95%CI -1.336 to -0.084, P = 0.026) and IFN-α2 (Hedges’ g -0.831; 95%CI -1.444 to -0.219, P = 0.008) (Fig. 2a). Then, the systematic review identified some underlying inflammatory markers reported in one study that were significantly changed in patients with PD, including elevated levels of IL-33, CCL18, Pentraxin 3 (PTX3), soluble vascular cell adhesion molecule-1(sVCAM-1), neutrophil gelatinase-associated lipocalin (NGAL), high mobility group 1 (HMGB1) and platelet-derived growth factor-B (PDGFB), as well as reduced levels of IL-3, IL-27, PDGF, β-nerve growth factor (NGF) and fibroblast growth factor (FGF)-basic (Fig. 3a). Other blood biomarkers that were altered in PD group are presented in Supplementary Figs. 1–2.
The peripheral blood (a) and cerebrospinal fluid (b) inflammatory markers with significant effect sizes (Hedges’ g) were displayed in comparisons for PD patients versus controls. Orange spots indicate Hedges’ g of each marker, and green and pink bars indicate the number of studies included. CCL chemokine (C-C motif) ligand, CRP C-reactive protein, CX3CL CX3 chemokine ligand, CXCL chemokine (C-X-C motif) ligand, IFN Interferon, IL interleukin, MCP monocyte chemoattractant protein, NT-pro BNP N-terminal pro-B-type natriuretic peptide, PD Parkinson’s disease, SDF stromal cell-derived factor, STNFR soluble tumour necrosis factor receptor, TNF tumour necrosis factor.
The peripheral blood (a) and cerebrospinal fluid (b) inflammatory markers with significant effect sizes (Hedges’ g) were displayed in comparisons for PD patients versus controls. Violet spots indicate Hedges’ g of each marker, and blue and orange bars indicate the number of studies included. CCL chemokine (C-C motif) ligand, CSF macrophage-colony stimulating factor, CXCL chemokine (C-X-C motif) ligand, FGF fibroblast growth factor, GRO growth-regulated oncogene, IL interleukin, HMGB high-mobility group box, MCP monocyte chemoattractant protein, MEC mucosae-associated epithelial chemokine, MIP macrophage inflammatory protein, NGAL neutrophil gelatinase-associated lipocalin, NGF nerve growth factor, PD Parkinson’s disease, PDGFB platelet-derived growth factor-B, PTX pentraxin, PD-L programmed death-ligand, SCF stem cell factor, SDF stromal cell-derived factor, sVCAM soluble vascular cell adhesion molecule, VEGF-A vascular endothelial growth factor A.
Comparisons of CSF biomarkers between PD patients and control
Random-effects meta-analyses also showed increased CSF levels of IL-6 (Hedges’ g 0.559; 95%CI 0.163 to 0.955, P = 0.006), TNF-α (Hedges’ g 0.599; 95%CI 0.023 to 1.175, P = 0.024), IL-1β (Hedges’ g 0.326; 95%CI 0.105 to 0.547, P = 0.004), CRP (Hedges’ g 1.231; 95%CI 0.321 to 2.141, P = 0.008), CCL2 (Hedges’ g 0.351; 95%CI 0.090 to 0.612, P = 0.008) and nitric oxide (NO; Hedges’ g 0.901; 95%CI 0.188 to 1.614, P = 0.013) (Fig. 2b). Moreover, lower concentrations of IL-16, IL-17A, CCL8, CCL23, CXCL1, β-NGF, FGF-19, stem cell factor (SCF), macrophage-colony stimulating factor (CSF-1), programmed death-ligand 1 (PD-L1) and vascular endothelial growth factor A (VEGF-A) were discovered in PD participants than in controls, whereas increased levels of CCL28 were detected. The nonsignificant markers in CSF for patients with PD were shown in Supplementary Figs. 3–4.
Publication biases and sensitivity analyses
Egger’s tests identified that publication biases were found for IL-6, CRP, IL-1β, IFN-γ and STNFR1 in peripheral blood (P < 0.050), as well as IL-6, TNF-α and NO in CSF. These findings suggested the data for these markers were not sufficiently robust. The conflicting findings among studies might be due to differences in assays used to detect cytokines and chemokines, such as conventional enzyme-linked immunosorbent assay (ELISA), multiplex cytokine panel and cytometric beads array (CBA). Then, the sensitivity analyses were employed to reduce these biases and subgroup analyses were performed according to assay types. On the one hand, random-effects meta-analyses showed that increased levels of TNF-α, IL-6, IL-1β, STNFR1and CRP among PD patients were identified in peripheral blood. The increased concentrations of IL-1β, IL-6, TNF-α, IL-4 and transforming growth factor (TGF)-β in CSF were identified using ELISA. Similarly, reduced levels of IFN-γ and IL-1 receptor antagonist (IL-1RA) in peripheral blood, as well as chitinase protein 40 (YKL-40) in CSF were observed (Fig. 4a). On the other hand, TNF-α, IL-8, CCL2 and CX3CL1 in blood were significantly elevated in subjects with PD as determined using multiplex panels. Additionally, increased IL-4 and decreased TGF-α levels were detected in CSF (Fig. 4b).
Significant comparisons of peripheral blood and CSF biomarkers using ELISA (a) and multiplex cytokine (b) are shown. Inflammatory markers with significant effect sizes (Hedges’ g) were displayed in comparisons of PD patients versus controls. Violet (a) and dark green (b) spots indicate Hedges’ g of each marker, and green (a) and pink (b) bars indicate the number of studies included. Abbreviations: CRP C-reactive protein, CSF cerebrospinal fluid, CX3CL CX3 chemokine ligand, ELISA enzyme-linked immunosorbent assay; IFN interferon, IL interleukin, IL-1RA IL-1 receptor antagonist, MCP monocyte chemoattractant protein, TGF transforming growth factor, TNF tumour necrosis factor, YKL chitinase-3-like protein.
Diagnostic accuracy of inflammatory biomarkers in the identification of PD
Single and combined markers of inflammation were used in the systematic review of 17 and 7 eligible studies, respectively. On the one hand, more than one study illustrated that CRP in peripheral blood, as well as soluble triggering receptor expressed on myeloid cells 2 (sTREM2), central nervous system specific protein beta (S100β) and YKL-40 in CSF, exhibited good diagnostic accuracy in distinguishing PD patients from controls. In addition, sVCAM-1, NOD-like receptor thermal protein domain associated protein 3 (NLRP3), IL-1β, CXCL12 and IL-8 in blood showed excellent diagnostic values (area under the curve [AUC] > 0.80), whereas PTX3, serum amyloid A (SAA) and CX3CL1 in blood, as well as amyloid precursor protein-alpha (sAPP-α), TNF-α and IL-6 showed moderate accuracy (AUC 0.60-0.80). On the other hand, the sensitivity and specificity of a single biomarker were insufficient based on the use of the reported assays, and the diagnostic accuracy was greatly enhanced upon the combined use of multiple markers. Specifically, inflammatory markers combined with α-synuclein, AD core biomarkers and basic characteristics yielded optimum values (Table 1).
Diagnostic values of inflammatory biomarkers based on PD clinical features
We enroled studies that investigated inflammatory markers in relation to clinical features of motor and nonmotor symptoms, and a detailed overview is displayed in Tables 2–3. First, the systematic review summarized 36 records. Several studies have confirmed that abnormal IL-6, CRP, TNF-α, IL-4, IL-8, and TGF-β levels were associated with worse motor function assessed by the Unified Parkinson’s Disease Rating Scale (UPDRS), whereas CRP and fractalkine might be potential markers of freezing of gait (FOG). Research on nonmotor symptoms included 48 studies that focused on cognitive impairment, depression and anxiety, sleep disorders, fatigue, neuropsychiatric symptoms and autonomic function. Studies have reported that IL-6, TNF-α, CRP, YKL-40, IL-17, IL-1β, CCL2, IL-2, and IL‐8 are related to worse cognitive function or cognitive deterioration, while CRP, TNF-α, sIL-2R and CCL2 reflect severe symptoms of depression and anxiety. Sleep disorders, including RBD and ESS, exhibit significantly altered levels of IL-6, CRP, IL-1β, sTREM2, CCL3 and NO, suggesting these markers represent potential markers in PD patients. In addition, some inflammatory markers were closely associated with fatigue, hallucinations and illusions.
Functional enrichment and protein‒protein interaction (PPI) network construction analyses
Based on the identified proteins, we conducted Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses to predict the potential function of robust markers. KEGG pathways with adjusted P < 0.05 were considered statistically significant. The results of KEGG pathway enrichment analysis showed that these markers were mainly involved in cytokine‒cytokine receptor interactions, human cytomegalovirus infection, rheumatoid arthritis, influenza A and the malaria pathway (Fig. 5a).
The KEGG pathway enrichment analysis showed that the inflammatory markers related to PD were mainly involved in cytokine–cytokine receptor interactions, human cytomegalovirus infection, rheumatoid arthritis, influenza A and the malaria pathway (a). The PPI analysis revealed that the major functions were involved in cytokine receptor binding, cytokine activity, leucocyte migration, chemokine receptor binding, myeloid leucocyte migration, cellular response to chemokine and leucocyte chemotaxis (b).
PPI network analysis was performed using the Search Tool for the Retrieval of Interacting Genes (STRING) to predict protein functional associations. The interaction network of overlapping targets with a combined score of >0.4 was considered statistically significant. Subsequently, the network was imported into Cytoscape software for visualization11. As shown in Fig. 5b, the network contains 37 nodes and 396 edges. The analysis revealed that the following functions were involved based on the 17 most significant targets: cytokine receptor binding, cytokine activity, leucocyte migration, chemokine receptor binding, myeloid leucocyte migration, cellular response to chemokine and leucocyte chemotaxis. Furthermore, the results of 39 potential markers are shown in Supplementary Fig. 2.
Discussion
Our meta-analysis comprehensively demonstrated multiple significant differences in inflammatory biomarker levels in peripheral blood and CSF between the PD and control groups. As noted, several potential markers were identified based on their ability to differentiate PD patients from healthy controls with good performance. Moreover, some of these inflammatory markers might represent biomarkers of clinical features, including motor and nonmotor symptoms. These findings suggested noteworthy blood and CSF alterations in inflammatory markers in PD patients, implying the important role of inflammation in PD pathology, and providing optimal biomarkers for the early disease diagnosis and monitoring.
To the best of our knowledge, this meta-analysis performed the most comprehensive evaluation to investigate the changes in peripheral inflammatory markers of PD. We found significant increases in inflammatory cytokine levels (IL-6, IL-1β and TNF-α) in both peripheral blood and CSF among patients with PD compared to healthy controls. These findings are consistent with previous meta-analyses9,10. Levels of the chemokine CCL2, also named monocyte chemoattractant protein-1 (MCP-1), which is associated with the recruitment of monocytes and T cells to sites of inflammation, were also increased in PD patients. However, inconsistent results have been reported in which only a few inflammatory markers showed significant differences in blood or CSF. We also found increased blood chemokine concentrations of CX3CL1 (fractalkine) and CXCL12 (stromal-derived factor [SDF]-1) as well as reduced cytokine levels of IL-4 and IFN-γ, in the PD group compared with the control group. In addition, IL-2 and CCL5 (RANTES) levels were previously reported to be elevated in patients with PD9, but significant differences were not observed in our study. This finding was likely attributed to the larger sample size and stricter inclusion criteria of this analysis. The increased levels of CRP in blood and CSF were verified in our study and a previous meta-analysis12, strengthening the clinical evidence that patients with PD exhibit increased inflammatory activation.
It has been verified that the expression and peripheral levels of proinflammatory cytokines and chemokines are significantly increased in patients with PD, which have been broadly documented to correlate with the hypothesis that α-synuclein in the brain directly activates microglia13. However, clinical alterations in these markers and their effects on PD progression are controversial. First, cytokines promote the apoptosis of neurons, oligodendrocytes and astrocytes, damage myelinated axons; and even initiate neuroprotective effects. These effects occur independent of the immunoregulatory properties of cytokines14. The most studied cytokines in PD are IL-6, IL-1β and TNF-α, and the role of IL-6 is distinct from that of IL-1β and TNF-α based on the contributions of its pro-and anti-inflammatory functions to neuropathology15,16. A previous study observed an increase in IL-6 in the SN region of the postmortem brain of PD patients17, and IL-6 plasma levels are also related to PD progression18. Similarly, a model of neuron cultures shows that chronic exposure to IL-6 during neuronal development can lead to cell damage and death in a subpopulation of developing granule neurons19. This meta-analysis discovered elevated peripheral levels of IL-6 in patients with PD. Thus, we suggest that enhanced circulating levels of IL-6 may be proinflammatory, leading to the progression of PD pathophysiology. On the other hand, the major proinflammatory factors IL-1β and TNF-α can induce oxidative stress, neuronal death and in particular the loss of dopaminergic neurons in PD20,21. It has been reported that sustained expression of IL-1β in the SN causes irreversible and pronounced dopaminergic neuronal loss and motor symptoms of PD22. Furthermore, treatments that reduce IL-1β and TNF-α levels may significantly improve motor function in PD mice23. TNF-α and its receptor sTNFR1, which regulate numerous physiological processes in the CNS, exacerbate the main pathological changes of PD (progressive loss of dopaminergic neurons) in vivo24. Our meta-analyses demonstrated notable differences in the peripheral concentrations of cytokines, implying that these markers might be useful in monitoring disease deterioration.
Second, only a few studies have evaluated circulating levels of chemokines in PD patients, and the results are inconclusive. Interestingly, elevated MCP-1 levels were found in the peripheral blood and CSF of patients with PD compared with controls according to our findings. MCP-1, one of the most highly and transiently expressed chemokines during inflammation, has been implicated in many neurodegenerative disorders through the regulation of monocyte chemotaxis and endothelial activation25. Preclinical studies in mouse models suggest that MCP-1 causes neuronal leakage through the blood-brain barrier (BBB) and macrophage polarization26 and promotes the continuous differentiation of dopamine precursors and neurogenesis of dopaminergic neurons in the midbrain27. Additionally, a clinical study illustrated a positive association between MCP-1 and nonmotor symptoms28. Other chemokines, such as fractalkine and SDF-1, are increased in the peripheral blood of PD subjects. Emerging evidence suggests the crucial role of fractalkine in neuron-to-glia communication signalling in PD29, and SDF-1 is correlated with the apoptosis of PD-related neurons by activating chemokine receptor 4 (CXCR4)30.In contrast to our findings, previous studies have reported that peripheral RANTES is significantly elevated and suggested that CCL5 produced from the CNS penetrates into the serum through the BBB31. These inflammatory targets provide further opportunities to explore their promising therapeutic values in PD.
Anti-inflammatory strategies are also considered beneficial for PD. Our results revealed controversial findings for the anti-inflammatory marker IL-4 in peripheral blood and CSF, possibly suggesting dual functions in the CNS. IL-4 shapes microglial functions to promotes the survival of dopaminergic neurons in animal models32, which underlines the therapeutic potential of IL-4 administration in PD. In addition, IL-4 promotes neurodegeneration in proinflammatory rat models by contributing to microglial activation, IL-1β production, and BBB disruption33. In addition, the peripheral levels of IFN-γ unexpectedly exhibited diverse alterations. Past studies reported that IFN-γ deficiency attenuated dopaminergic lesions in PD models by inhibiting microgliosis and inducible NO synthase (iNOS) expression, indicating that IFN-γ may contribute to dopaminergic loss by acting through microglial activation34,35. However, IFN-γ increases the proliferation of neural precursor cells and enhances neurogenesis in AD models36. Current studies do not entirely disclose how peripheral markers of inflammation reflect neuroinflammation activity. Hence, the inconsistent results for these markers in the CNS and peripheral blood system urgently need to be explored in future studies.
Most of the studies have consistently demonstrated obviously increased CRP levels both in blood and CSF in patients with PD. Some scholars hold the view that CRP can also be generated by neurons and microglia in the CNS37, and epidemiological studies observe that long-term anti-inflammatory medication therapy is beneficial and will delay or prevent dopaminergic cell death by inhibiting the proinflammatory responses of microglia38. However, others believe that patients with PD are more susceptible and have a higher infectious burden than health individuals39. Taken together, the present analyses cannot completely determine the actual mechanisms of these proteins in PD initiation and progression.
The network construction assists us better understand the interaction among inflammatory markers and aim at fresh therapeutic targets of PD. For instance, the NF-κB pathway participates in microglia activation and consequently gives rise to the release of multiple pro-inflammatory and anti-inflammatory cytokines40, and can subsequently release chemokines and recruit peripheral immune cells, indicating the joint effort of cytokines and chemokines of inflammation in PD. The inflammatory markers also take part in other immune reaction like leucocyte migration and leucocyte chemotaxis41, which reflects the diverse function of them.
Given the variety of studies included in this meta-analysis, it is inevitable that each cytokine will exhibit heterogeneity. However, techniques are currently being developed achieve greater sensitivity, and ultrasensitive platforms, including Luminex XMAP, Meso Scale Discovery (MSD) and Simoa (Single Molecular Array), have appeared. These platforms facilitate the detection of multiple markers in the same sample and overcome issues associated with low levels of target biomarkers. Here, we conducted subgroup analyses based on detection techniques to adjust for potential confounders. However, the results were not consistent with our expected findings for the combined data for inflammatory markers measured by multiplex assays, as obvious heterogeneity remained. We hypothesize that these discrepancies are partly attributed to the sensitivities of the various assays used and patient characteristics.
Inflammation can also reflect more advanced motor and nonmotor symptom processes. We conclude that a number of inflammatory markers in blood and CSF are associated with more severe motor and nonmotor symptoms, whereas some are able to predict symptomatic progression. Exploring the diagnostic and prognostic values of inflammatory markers for clinical symptoms is essential but still inadequate; therefore, future research may pay more attention to the clinical features of PD to enrich maximize the therapeutic benefit. In addition, the combined diagnosis is augmented largely by the use of multiple cytokines and chemokines, such as α-synuclein and AD core biomarkers, as well as the type variances. These results imply that multiplex assays measuring various inflammatory markers can serve as appropriate detection approaches.
Limitations to our meta-analysis should be noted. The foremost weakness is the lack of relative studies for some newly identified markers. Due to the limited availability of information, this study is underpowered to investigate alterations in these inflammatory markers in PD. Thus, future studies should better address these aspects. Next, large differences were noted based on measurement approaches, so multiplex assays should be validated in larger cohorts and more unified operating platforms should be employed. Finally, certain eligible articles and inflammatory markers might be missed even though systemic research was performed, and a portion of the articles identified reported results in the form that was inappropriate for the present meta-analysis, which would potentially bias our results.
In summary, our meta-analysis demonstrated altered IL-6, TNF-α, IL-1β, MCP-1 and CRP levels in both peripheral blood and CSF in PD patients versus control groups, and altered IL-4, IFN-γ, STNFR1 and fractalkine only in blood. These findings based on a large sample size strengthen the clinical evidence that PD is accompanied by a specific peripheral inflammatory response.
Methods
Search strategy and selection criteria
This systematic review and meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) 2009 guidelines (Supplementary Table 6)42. Electronic databases (PubMed, Cochrane Library, Embase and Web of Science) were systematically searched for studies that reported data of inflammatory biomarkers in peripheral blood and CSF for patients with PD versus controls from database inception to June 8, 2022. The initial study protocol was preregistered at PROSPERO (CRD42022349182). The full search strategy is listed in Supplementary Table 7, and additional literature was added by hand-searching references of relevant reviews and meta-analyses.
Studies were included if they met the following criteria: (a) original studies reported data about concentrations of inflammatory markers in at least two of the groups (PD and control); (b) literature sources and necessary data were met; and (c) the principles of PD diagnosis were qualified. Studies were excluded for the following reasons: (a) measured marker concentrations in postmortem samples, animals or in vitro; (b) duplicated samples that overlapped with other studies; and (c) raw data could not be obtained completely. For several publications reported from the same centre, we included the publication that had greatest sample size.
Data extraction
Data including study characteristics (i.e., first author, publication year, study design, sample size, age, sex and region), information for potential moderator analysis (i.e., sample sources and assay types) and PD assessments (i.e., diagnostic criteria, disease duration, Hoehn-Yahn stages and UPDRS III scores), were independently extracted by two researchers. Biomarkers are presented as concentrations with the mean (SD [standard deviation]), median (IQR [interquartile range]) or median (range), and the data of the latter is converted to the former by using a new evaluative method43. All data and any controversies were checked and resolved by a third author.
Quality assessment of studies
The Newcastle‒Ottawa Scale (NOS) was used for quality assessments of all potentially eligible studies44. The scale ranges from 0 to 9 stars and awards four stars for selection of study participants, two stars for comparability of studies, and three stars for the adequate ascertainment of outcomes. Studies with NOS scores <6 were recognized to be of low quality and therefore excluded.
Statistical analysis
All statistical analyses were conducted using Comprehensive Meta-Analysis Software (version 3) and GraphPad Prism (version 8). Effect sizes (ESs) were primarily adopted from sample sizes and mean (SD) values of cytokine concentrations between patients with PD and controls. Additionally, ESs were calculated from sample size and P-values if mean (SD) data were not available. Hedges’ g values were performed as the combined ESs to reduce the potential biases45, and random effects meta-analysis was used in all analyses. Heterogeneities among studies were assessed using the Cochrane Q test and I2 index. P < 0.10 indicated a significant difference for the Cochrane Q, and I2 index values 0.25, 0.50, and 0.75 distinguished small, moderate, and high levels of heterogeneity, respectively. Publication bias was conducted to assess if whether the pooled effect values were impacted by parts of the studies’ positive results and assessed by Egger’s test (>3 studies). Then, subgroup analysis was employed to significantly reduce the heterogeneity and publication bias within every subgroup. In addition, inflammatory markers measured in one study were assessed qualitatively in the systematic review. P-values of 0.05 or less were considered significant.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
All data generated or analysed during this study are included in this published article (and its supplementary information files).
References
Jankovic, J. Parkinson’s disease: clinical features and diagnosis. J. Neurol. Neurosurg. Psychiatry 79, 368–376 (2008).
Mazzoni, P., Shabbott, B. & Cortés, J. C. Motor control abnormalities in Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2, a009282 (2012).
Chaudhuri, K. R. & Schapira, A. H. Non-motor symptoms of Parkinson’s disease: dopaminergic pathophysiology and treatment. Lancet Neurol. 8, 464–474 (2009).
Wang, Q., Liu, Y. & Zhou, J. Neuroinflammation in Parkinson’s disease and its potential as therapeutic target. Transl. Neurodegener. 4, 19 (2015).
Whitton, P. S. Inflammation as a causative factor in the aetiology of Parkinson’s disease. Br. J. Pharm. 150, 963–976 (2007).
Tabas, I. & Glass, C. K. Anti-inflammatory therapy in chronic disease: challenges and opportunities. Science 339, 166–172 (2013).
Mosley, R. L., Hutter-Saunders, J. A., Stone, D. K. & Gendelman, H. E. Inflammation and adaptive immunity in Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2, a009381 (2012).
Zimmermann, M. & Brockmann, K. Blood and cerebrospinal fluid biomarkers of inflammation in Parkinson’s disease. J.Parkinson’s Dis. https://doi.org/10.3233/jpd-223277 (2022).
Qin, X. Y., Zhang, S. P., Cao, C., Loh, Y. P. & Cheng, Y. Aberrations in peripheral inflammatory cytokine levels in Parkinson disease: a systematic review and meta-analysis. JAMA Neurol. 73, 1316–1324 (2016).
Chen, X., Hu, Y., Cao, Z., Liu, Q. & Cheng, Y. Cerebrospinal fluid inflammatory cytokine aberrations in Alzheimer’s disease, Parkinson’s disease and amyotrophic lateral sclerosis: a systematic review and meta-analysis. Front. Immunol. https://doi.org/10.3389/fimmu.2018.02122 (2018).
Shannon, P. et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 13, 2498–2504 (2003).
Qiu, X. et al. C-Reactive protein and risk of Parkinson’s disease: a systematic review and meta-analysis. Fronti. Neurol. https://doi.org/10.3389/fneur.2019.00384 (2019).
Béraud, D. et al. Microglial activation and antioxidant responses induced by the Parkinson’s disease protein α-synuclein. J. NeuroImmune Pharmacol.: Off. J. Soc. NeuroImmune Pharmacol. 8, 94–117 (2013).
Tansey, M. G. et al. Neuroinflammation in Parkinson’s disease: is there sufficient evidence for mechanism-based interventional therapy. Front. Biosci. 13, 709–717 (2008).
Chen, H. L., O’Reilly, E. J., Schwarzschild, M. A. & Ascherio, A. Peripheral inflammatory biomarkers and risk of Parkinson’s disease. Am. J. Epidemiol. 167, 90–95 (2008).
Bagli, M. et al. Polymorphisms of the gene encoding the inflammatory cytokine interleukin-6 determine the magnitude of the increase in soluble interleukin-6 receptor levels in Alzheimer’s disease. Eur. Arch. Psychiatry Clin. Neurosci. 253, 44–48 (2003).
Mogi, M. et al. Interleukin-1 beta, interleukin-6, epidermal growth factor and transforming growth factor-alpha are elevated in the brain from parkinsonian patients. Neurosci. Lett. 180, 147–150 (1994).
Selikhova, M. V., Kushlinskii, N. E., Lyubimova, N. V. & Gusev, E. I. Impaired production of plasma interleukin-6 in patients with Parkinson’s disease. Bull. Exp. Biol. Med 133, 81–83 (2002).
Conroy, S. M. et al. Interleukin-6 produces neuronal loss in developing cerebellar granule neuron cultures. J. Neuroimmunol. 155, 43–54 (2004).
Ye, L. et al. IL-1β and TNF-α induce neurotoxicity through glutamate production: a potential role for neuronal glutaminase. J. Neurochem 125, 897–908 (2013).
Dai, D. et al. Association of NQO1 and TNF polymorphisms with Parkinson’s disease: a meta-analysis of 15 genetic association studies. Biomed. Rep. 2, 713–718 (2014).
Ferrari, C. C. et al. Progressive neurodegeneration and motor disabilities induced by chronic expression of IL-1beta in the substantia nigra. Neurobiol. Dis. 24, 183–193 (2006).
Manocha, G. D. et al. Defining the contribution of neuroinflammation to Parkinson’s disease in humanized immune system mice. Mol. Neurodegener. 12, 17 (2017).
Chertoff, M. et al. Neuroprotective and neurodegenerative effects of the chronic expression of tumor necrosis factor α in the nigrostriatal dopaminergic circuit of adult mice. Exp. Neurol. 227, 237–251 (2011).
Bose, S. & Cho, J. Role of chemokine CCL2 and its receptor CCR2 in neurodegenerative diseases. Arch. Pharmacal Res. 36, 1039–1050 (2013).
Sawyer, A. J. et al. The effect of inflammatory cell-derived MCP-1 loss on neuronal survival during chronic neuroinflammation. Biomaterials 35, 6698–6706 (2014).
Edman, L. C., Mira, H. & Arenas, E. The beta-chemokines CCL2 and CCL7 are two novel differentiation factors for midbrain dopaminergic precursors and neurons. Exp. Cell Res. 314, 2123–2130 (2008).
Lindqvist, D. et al. Cerebrospinal fluid inflammatory markers in Parkinson’s disease-associations with depression, fatigue, and cognitive impairment. Brain, Behav., Immun. 33, 183–189 (2013).
Angelopoulou, E., Paudel, Y. N., Shaikh, M. F. & Piperi, C. Fractalkine (CX3CL1) signaling and neuroinflammation in Parkinson’s disease: Potential clinical and therapeutic implications. Pharm. Res. 158, 104930 (2020).
Liu, J. Q., Chu, S. F., Zhou, X., Zhang, D. Y. & Chen, N. H. Role of chemokines in Parkinson’s disease. Brain Res. Bull. 152, 11–18 (2019).
Gangemi, S. et al. Effect of levodopa on interleukin-15 and RANTES circulating levels in patients affected by Parkinson’s disease. Mediators Inflamm. 12, 251–253 (2003).
Hühner, L. et al. Interleukin-4 protects dopaminergic neurons in vitro but is dispensable for MPTP-induced neurodegeneration in vivo. Front Mol. Neurosci. 10, 62 (2017).
Bok, E., Cho, E. J., Chung, E. S., Shin, W. H. & Jin, B. K. Interleukin-4 contributes to degeneration of dopamine neurons in the lipopolysaccharide-treated substantia Nigra in vivo. Exp. Neurobiol. 27, 309–319 (2018).
Mangano, E. N. et al. Interferon-γ plays a role in paraquat-induced neurodegeneration involving oxidative and proinflammatory pathways. Neurobiol. Aging 33, 1411–1426 (2012).
Mount, M. P. et al. Involvement of interferon-gamma in microglial-mediated loss of dopaminergic neurons. J. Neurosci.: Off. J. Soc. Neurosci. 27, 3328–3337 (2007).
Baron, R. et al. IFN-gamma enhances neurogenesis in wild-type mice and in a mouse model of Alzheimer’s disease. Faseb j. 22, 2843–2852 (2008).
Yasojima, K., Schwab, C., McGeer, E. G. & McGeer, P. L. Human neurons generate C-reactive protein and amyloid P: upregulation in Alzheimer’s disease. Brain Res 887, 80–89 (2000).
Rees, K. et al. Non-steroidal anti-inflammatory drugs as disease-modifying agents for Parkinson’s disease: evidence from observational studies. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD008454.pub2 (2011).
Bu, X. L. et al. The association between infectious burden and Parkinson’s disease: a case-control study. Parkinsonism Relat. Disord. 21, 877–881 (2015).
Lee, M. Neurotransmitters and microglial-mediated neuroinflammation. Curr. protein Pept. Sci. 14, 21–32 (2013).
Konstantin Nissen, S. et al. Changes in CD163+, CD11b+, and CCR2+ peripheral monocytes relate to Parkinson’s disease and cognition. Brain, Behav., Immun. 101, 182–193 (2022).
Liberati, A. et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 339, b2700 (2009).
Luo, D., Wan, X., Liu, J. & Tong, T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat. methods Med. Res. 27, 1785–1805 (2018).
Wells, G. et al. The Newcastle–Ottawa Scale (NOS) for assessing the quality of non-randomized studies in meta-analysis. Eur. J. Epidemiol. 25, 603–605 (2000).
Grissom, R. & Kim, J. Effect Sizes for Research: A Broad Practical Approach (Informa UK Limited, 2005).
Acknowledgements
This study was funded by the National Natural Science Foundation of Hubei Province (grant number 2020CFB590) and the Fundamental Research Funds for the Central Universities (YCJJ202201020).
Author information
Authors and Affiliations
Contributions
Z.X., Jingyi Li and Y.Q. designed and conceptualized the study; Y.Q., Jiangting Li, Q.Q. and D.W. conducted the study. Y.Q., J.Z. and K.A. analysed and extracted the data. Y.Q., Z. Mao., Y.X. and Z. Min. wrote the first draft of the manuscript. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Qu, Y., Li, J., Qin, Q. et al. A systematic review and meta-analysis of inflammatory biomarkers in Parkinson’s disease. npj Parkinsons Dis. 9, 18 (2023). https://doi.org/10.1038/s41531-023-00449-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41531-023-00449-5