Abstract
While therapies such as chemotherapy combined with immunotherapy, sacituzumab govitecan, and PARP inhibitors are available for metastatic TNBC, on disease progression after these therapies, the mainstay of therapy is chemotherapy. Apatinib is a small-molecule tyrosine kinase inhibitor that has promising anti-angiogenesis and antitumor activity for TNBC. We aimed to evaluate the safety and efficacy of adding apatinib to chemotherapy in patients with advanced TNBC with failed first/second-line treatment. A total of 66 patients were randomly assigned, in a 1:1 ratio, to receive vinorelbine or vinorelbine with apatinib in 28-day cycles. The primary endpoint was progression-free survival (PFS). Secondary endpoints included overall survival (OS), overall response rate (ORR) and safety. 33 received apatinib plus vinorelbine and 32 received vinorelbine (1 was withdrawal). Median PFS was significantly longer in the apatinib plus vinorelbine group than in the vinorelbine group (3.9 months vs. 2.0 months; hazard ratio, 1.82; 95% confidence interval [CI], 1.06 to 3.11; P = 0.026). Median OS was 11.5 months with apatinib plus vinorelbine and 9.9 months with vinorelbine (HR,1.01; 95% CI, 0.51 to 1.97; P = 0.985). The ORR was 9.1% in the apatinib plus vinorelbine group and 6.3% in the vinorelbine group (P = 0.667). The most common treatment-related hematologic grade 3–4 adverse events in apatinib plus vinorelbine group, were leukopenia, granulocytopenia, anemia, and thrombocytopenia. no treatment-related nonhematologic grade 4 adverse events or treatment-related deaths were observed. Collectively, adding apatinib to vinorelbine shows a promising benefit in PFS compared to vinorelbine monotherapy, with an excellent toxicity profile, warranting further exploration.
Similar content being viewed by others
Introduction
Breast cancer is the most frequently diagnosed malignancy among female worldwide and is a highly heterogeneous disease with diverse molecular profiles, which is closely related to prognosis and treatment response1,2. Triple-negative breast cancer (TNBC) accounts for 12–20% of all invasive breast cancer cases. It is characterized by negative estrogen receptor (ER), progesterone receptor (PR), and nonamplified human epidermal growth factor receptor 2 (HER2)3,4. Compared with other subtypes, TNBC is characterized by high histological grade, early onset (<50 years), malignancy aggressiveness, strong invasion, high risk of postoperative recurrence and metastasis, high probability of visceral and brain metastasis, rapid disease progression, and poor clinical prognosis5.
Chemotherapy remains the mainstay of treatment, which is limited by short duration of response and considerable toxicity. Although the combination of chemotherapy and immunotherapy or PARP inhibitors prolonged the survival to a certain extent, but the results for patients with metastatic TNBC remain significantly poor compared to other subtypes6,7,8,9,10. No standard treatment exists for TNBC with failure of multi-line therapies. there is no clear favored sequencing of agents after the first few lines—there are several standard therapies for metastatic TNBC. Novel treatment approaches that target this population of patients are desperately needed.
Anti-angiogenic drugs have been gradually attempted in the treatment of TNBC due to its higher expression of VEGF and VEGFR compared with other subtypes of breast cancer, despite the side effects of bleeding, hypertension, and thrombus11,12,13. Since 2009, several phase III trials have investigated anti-angiogenesis molecular targeted therapies in metastatic TNBC14,15,16. Randomized clinical trials in metastatic breast cancer document that the addition of bevacizumab to chemotherapy agents modestly improves time to progression and response rates17,18,19. The time to progression impact may vary among cytotoxic agents and appears greatest with bevacizumab in combination with weekly paclitaxel20. Besides, none of these studies demonstrates an increase in OS or Quality of life (QOL) when analyzed alone or in a meta-analysis of the trails21. Although bevacizumab does not benefit PFS conversion to OS or QOL, anti-angiogenic drugs remain an important part of the treatment of metastatic breast cancer. The attempt of small molecule anti-angiogenesis inhibitors is also worthy of further exploration. Apatinib is a novel small-molecule tyrosine kinase inhibitor targeting vascular endothelial growth factor receptor-2, which opens a new era of oral targeted anti-angiogenesis therapy22,23,24,25. In our previous clinical study NCT01176669, we evaluated the optimum dose level for the efficacy and safety of apatinib monotherapy in heavily pretreated patients with metastatic TNBC in China. The results showed that apatinib (at a dose of 500 mg once daily) showed promising efficacy and safety in salvage treatment of TNBC. The median PFS was 3.3 months (95% CI, 2.2–4.5), and the median OS was 11.1 months (95% CI, 5. 1–17. 2), which was superior to sunitinib26.
With the increasing use of anthracyclines and taxanes in adjuvant and neoadjuvant treatment of early breast cancer, the range of effective chemotherapy drugs for advanced TNBC is further limited27. A considerable number of patients have received capecitabine or cisplatin during first few lines. Thus, vinorelbine is one of the few posterior line drugs that can be selected. Certain efficacy of vinorelbine had been observed in previous clinical practices, which can be set as the control group (25 mg/m2, intravenous drip, 1, 8, 15 days)28. According to the viewpoint of tumor vascular normalization: there exists a time window for normalizing the structure and function of tumor blood vessels after anti-angiogenic therapy, during which the sensitivity of tumor to chemoradiotherapy increases29,30. Combined with our previous clinical trials NCT01176669, we confirmed the efficacy and safety of apatinib monotherapy in heavily pretreated patients with metastatic TNBC in our center. However, there is no published literature on chemotherapy combined with apatinib.
The NAN trial, a prospective, open label, phase II multicenter trial, was designed to further investigate whether apatinib administration before chemotherapy (apatinib 250 mg oral, qd, days 1–5, 8–12, 15–19) can increase the efficacy of vinorelbine by taking advantage of the time window of vascular normalization in women with advanced TNBC.
Result
Patients
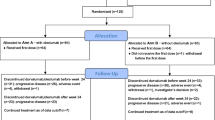
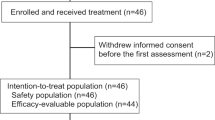
Between September 14, 2017 and December 08, 2020, a total of 66 patients underwent randomization. 33 received apatinib plus vinorelbine treatment, 32 patients who were randomly assigned to vinorelbine monotherapy and 1 patient withdrew consent without receiving treatment (Fig. 1). Demographic distributions and clinicopathologic characteristics of the enrolled patients are summarized in Table 1. The mean age of included patients at the time of diagnosis was 48 years. All the patients were diagnosed with invasive ductal carcinoma with metastatic disease. Visceral metastatic rate in vinorelbine monotherapy group and apatinib plus vinorelbine group was 19% and 27%, respectively. Accordingly, 15% and 19% of patients had metastases in ≥3 organs. A majority of patients had received prior anthracycline or paclitaxel-based adjuvant or neoadjuvant chemotherapy and platinum-based metastatic chemotherapy. The median duration of follow-up was 22.3 months (range, 1.8–41.2). The data cutoff date was January 31, 2021.
A total of 66 patients underwent randomization. 33 received apatinib plus vinorelbine treatment, 32 patients who were randomly assigned to vinorelbine monotherapy and 1 patient withdrew consent without receiving treatment. Vinorelbine monotherapy was administered as a once-per-week intravenous infusion of 25 mg/m2 on days 1, 8, and 15 of each 28-day treatment cycle. In vinorelbine plus apatinib group, vinorelbine in administered intravenously at a dose of 20 mg/m2 on day 7, 14, and 21 of each 28-day treatment cycle. Apatinib 250 mg was administered orally once daily at a fixed time each day about 30 min after a meal, with treatment on days 1 to 5 of weeks 1, 2, and 3 within each 28-day cycle (if tolerable, the second cycle started with 500 mg per day).
Efficacy
We conducted survival analysis to evaluate the two groups on PFS by Kaplan‐Meier method. As shown in Fig. 2, Median PFS was significantly longer in the apatinib plus vinorelbine group than in the vinorelbine group (3.9 months vs. 2.0 months; hazard ratio for disease progression or death, 1.82; 95% confidence interval [CI], 1.06–3.11; P = 0.026). A total of 12.1% of the patients in the apatinib plus vinorelbine group and 15.6% of the patients in the vinorelbine group and did not have disease progression at data cutoff. Subgroup analysis of progression-free survival in the vinorelbine group and the apatinib plus vinorelbine group is summarized in Fig. 3. After analyzing with clinically relevant factors including age, menopausal status, number of metastases, previous treatment, visceral metastasis, our data showed that the risk of disease progression in apatinib plus vinorelbine group was apparently lower than that of patients in vinorelbine group.
At the time of data cutoff, 34 patients had died (52.3%): 20 (60.6%) in the apatinib plus vinorelbine group and 14 (43.8%) in the vinorelbine group. Median OS was 11.5 months with apatinib plus vinorelbine and 9.9 months with vinorelbine (HR,1.01; 95% CI, 0.51 to 1.97; P = 0.985). (Fig. 4). According to RECIST 1.1, we investigated the objective response rate (ORR) between the two groups, and the results revealed that the ORR was 9.1% among patients who received apatinib plus vinorelbine and 6.3% among those who received vinorelbine monotherapy (P = 0.667). Two patients in each group achieved a partial response (PR). As compared with no patients in the vinorelbine group, one (3.0%) of 33 patients in apatinib plus vin orelbine group achieved a complete response (CR), with the maximum diameter of the target lesion reducing from 18.3 mm at baseline to 4.9 mm after 4 cycles of therapy, this patient maintained a CR for 8.6 months (Table 2). At the time of the last follow-up, there were one patient maintaining PR status in apatinib plus vinorelbine group.
Safety
All 65 patients received at least one cycle of apatinib plus vinorelbine or vinorelbine monotherapy and the median treatment duration was 1.6 months (range, 0.4 to 12.7) in the apatinib plus vinorelbine group and 1.4 months (range, 0.3–9.7) in the vinorelbine group. Three patients in each group were still receiving study treatment at the data cutoff date (January 2021). Adverse events of any grade are summarized in Table 3. The incidence of hematological toxicity was higher in the apatinib plus vinorelbine group, Grade 3 or 4 hematologic adverse events occurred in those treated with apatinib plus vinorelbine versus vinorelbine, respectively, were leukopenia (42.4% vs. 40.6%), granulocytopenia (57.6% vs. 31.3%), anemia (9.1% vs. 12.5%) and thrombocytopenia (3.0% vs. 3.1%). The most common nonhematologic toxicities were increased ALT (54.5% vs.37.5%), fatigue (27.3% vs.18.8%), nausea (18.2% vs.18.8%) and hand–foot syndrome (24.2% vs.15.6%), which occurred more frequently in apatinib plus vinorelbine group than vinorelbine group. the majority of nonhematologic adverse events in the vinorelbine group were grade 1–2 in severity.
Most notably, the incidence of adverse events related to apatinib: hypertension and proteinuria were 15.2% and 3% respectively. No treatment-related nonhematologic grade 4 adverse events or treatment-related deaths were observed. Dose modification (reduction or interruption temporarily) was most commonly due to thrombocytopenia in the vinorelbine group (3.1%) and to leukopenia, granulocytopenia in the apatinib plus vinorelbine group (15.2%). Adverse events resulting in discontinuation of the drug occurred in 1(9%) of 33 patients who received apatinib plus vinorelbine group.
Discussion
This randomized, open-label, phase 2 trial is the first study to uncover the efficacy and safety of adding apatinib to chemotherapy in patients with advanced TNBC with failed first/second-line treatment. In line with our expectations, we demonstrated that apatinib plus vinorelbine revealed a longer PFS compare with those who received vinorelbine monotherapy (3.9 months vs. 2.0 months; P = 0.026). Furthermore, subgroup analysis showed that the addition of apatinib resulted in apparently lower the risk of disease progression among clinically relevant factors including age, menopausal status, number of metastases, previous treatment, visceral metastasis. This is statistically significant but be honest that it is clinically not as significant but how these results could inform future trials. Besides, it must be confessed, added toxicity for a 1.9 month benefit may not be worth it for some patients. Therefore, as a clinician, this regimen should also be selected after balancing radiotherapy and toxicity.
The secondary end point of OS indicated a tendency toward beneficial to survival in the patients with apatinib plus vinorelbine compared to vinorelbine despite statistically insignificant (11.5 months vs. 9.9 months; P = 0.985), which could be attribute to the insufficient follow-up time, the end point of death in many patients did not occur at the cut-off time point. Thus, further subgroup analysis among clinically relevant factors in patients will be launched after the overall survival data are mature. Although no significant difference in ORR was observed between apatinib plus vinorelbine treatment and vinorelbine monotherapy, but apatinib plus vinorelbine showed superior over vinorelbine with 9.1% vs. 6.3%, respectively. It is important to note that one (3.0%) of 33 patients in apatinib plus vinorelbine group achieved a CR and two (6.1%) achieved a partial response.
The combination of apatinib plus vinorelbine was considered reasonably well tolerated. Most frequent hematologic grade 3–4 adverse events were leukopenia and granulocytopenia, which was clinically controllable, and rapidly alleviate after dose modification. Since the dose escalation of apatinib (Initial dose: 250 mg; if tolerable, the second cycle: 500 mg), the common toxicity of apatinib: hand–foot syndrome, hypertension and proteinuria were mainly grade 1–2, only one patient developed grade 3 foot syndrome and proteinuria and recovery through dose reduction. Thus, apatinib related adverse events were generally administrable after appropriate clinically intervention. Furthermore, the design of 5-day continuous administration and 2-day rest brought benefits to the recovery of adverse effects of apatinib, thus increasing the tolerance of the drug.
Apatinib as an orally administered antiangiogenic drug, can make the existing tumor vessels degenerate, block the transport of oxygen and other nutrients for tumor growth and inhibit tumor neovascularization, thereby repress metastasis31. Importantly, apatinib can normalize the surviving tumor vessels, improve the delivery of chemotherapy drugs through reducing the pressure between tumor tissues, and enhance the efficacy of chemotherapy32. Recently, the efficacy of apatinib in advanced TNBC with failure of chemoradiotherapy has been confirmed in several studies33,34,35. In a retrospective study, combination of apatinib and capecitabine achieved a better PFS and tolerable toxicity compare with capecitabine monotherapy, which may regard as one of the options of the third-line treatment for advanced TNBC36. In previous NCT 01176669 trial, we evaluated the optimum dose level for the efficacy and safety of apatinib monotherapy in patients with metastatic TNBC. The results indicated that the lower daily dose of apatinib 500 mg/day is active in pretreated metastatic TNBC with perspective CRR, clinical benefit rate and PFS. NAN trail is an extension and replenishment to our previous NCT 01176669 trial, which fully utilized the interval of vascular normalization that may be brought by the administration of apatinib before chemotherapy, so as to maximize the efficacy of vinorelbine. Furthermore, we will further establish a subgroup of metronomic regimen chemotherapy to explore the effect of apatinib combined metronomic regimen on relieving toxicity on the basis of ensuring the curative effect.
Cytotoxic chemotherapy remains the chief treatment for metastatic TNBC as currently there are no endocrine or specific targeted regimes available37. Anthracyclines and taxanes are frequently used in the first-line or second-line treatment, the choice of drugs for back-line treatment has encountered a bottleneck38. A considerable number of patients have received capecitabine in clinical trials during first few lines39,40,41. Besides, the efficacy of xeloda is uncertain in patients who have relapsed after participating in clinical trials. Therefore, capecitabine was difficult to be chose as a chemotherapy drug in patients with advanced TNBC with failed first/second-line treatment. While, vinorelbine is a commonly used drug for posterior line rescue treatment with response rates of 36–50%42. Vinorelbine, a semisynthetic alkaloid from primula oblongata, is a cell cycle-specific drug, which can stop cell division in the metaphase of mitosis by interfering with the polymerization of tubulin43. Multiple clinical trials have explored the efficacy of vinorelbine-based later-line setting in metastatic TNBC44,45,46,47,48,49. Our previously prospective phase II trial also indicated that biweekly vinorelbine and oxaliplatin regimen is effective and well-tolerated as second- or third-line treatment for patients with metastatic TNBC28. In NAN study, the dose of vinorelbine combined with apatinib can refer to a phase I/II study of vinorelbine combined with sorafenib50. Besides, the side effects of apatinib need time to recover in order to increase drug tolerance, so compare with navelbine monotherapy arm, we conducted the dose reduction of navelbine.
Multiple targeted therapies have achieved drastic improvements in the treatment of metastatic TNBC. Recently clinical studies showed that adding programmed death receptor 1 (PD-1) or its ligand (PD-L1) blockade to chemotherapy significantly improved PFS in PD-L1 positive mTNBC patients51,52,53. PARP inhibitors -Olaparib and talazoparib are approved as a standard of care for the treatment of metastatic TNBC harboring a germline BRCA mutations54,55,56. Furthermore, antibody-drug conjugates (ADC) drugs including sacituzumab govitecan demonstrated high activity in pretreated mTNBC in a randomized phase III trial versus single-agent chemotherapy57,58.
Unfortunately, no approval has been given for failed multiple lines treatment on checkpoint inhibitors for TNBC in China. However, the clinical trials of PD1 in the treatment of metastatic breast cancer have been carried out, and we believe that data support will be available in the near future59,60,61. In view of the literature reports that there may be synergy between PD1 and small molecule antiangiogenic drugs62. Besides, the efficacy of vinorelbine combine with immunotherapy or PARP inhibitors in metastatic TNBC is not fully understood. Therefore, the optimal setting for combining apatinib plus vinorelbine therapy with multiple targeted therapies in patients with advanced TNBC is worth of further exploration.
In agreement with the above data, several limitations must be taken into account. The main limitation is considered to be the insufficient follow‐up time. On the basis of the OS Kaplan‐Meier curves, there remains more than 25% of the patients survived at the end of the follow‐up; the shortest follow-up time was only about 2 months. thus, the outcomes seem less rigorous. Moreover, owing to one patient withdrew consent without receiving treatment, the uneven distribution of patients’ number comes up with a relative basis outcome. Furthermore, in an effort to strengthen and extend above findings, the need for detecting VEGFR tissue biomarker with more survival prognosis outcomes should be launched to further confirm prognosis effectiveness of apatinib plus vinorelbine regime.
In conclusion, our study has elucidated that among patients with advanced TNBC with failed first/second-line treatment, adding apatinib to vinorelbine shows a promising benefit in PFS compared to vinorelbine monotherapy, with an excellent toxicity profile. Trails with a large sample sets and longer follow-up period warranting further exploration.
Method
Patients
NAN was a prospective, open label, single-center randomized phase II trial performed in Fudan University Shanghai Cancer Center. Eligible patients were female patients aged 18–70 years, with histologically confirmed recurrent (unresectable) or metastatic TNBC (defined as, ER/PR stain of <1% positive tumor cells with nuclear staining on immunohistochemistry [IHC] and negative HER2 status, defined as 0 or 1+ intensity on IHC, Patients with IHC 2+ were selected to have a fluorescent in situ hybridization test for HER2 gene amplification and the result is negative). Patients were required to have at least one measurable extracranial lesion according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1, an Eastern Cooperative Oncology Group (ECOG) performance status of 0–1 and received maximum of two prior chemotherapy regimens and met the following definitions of treatment failure: progression during the first-line or second-line treatment, or the interval between the follow-up disease progression and the last treatment <3 months. Previous radiotherapy within 4 weeks before enrollment was not permitted. Adequate hematologic (Hb ≥ 90 g/L, No blood transfusion within 14 days; ANC ≥ 1.5 × 109/L; PLT ≥ 75 × 109/L), hepatic (TBIL ≤ 1. 5 × ULN (Upper limit of normal value); ALT and AST ≤ 3 × ULN), and renal (Cr ≤ 1 × ULN) function and a life expectancy of at least 3 months were also required.
Patients with a history of treatment with the vinorelbine, or a history of treatment with the VEGFR tyrosine kinase inhibitors except bevacizumab, any factors interfering with oral medication, a history of psychotropic drug abuse and inability to abstain or mental disorders, any previous clinical trials within 4 weeks before starting NAN trail and women who were pregnant or lactating were excluded. Patients with brain metastases were also excluded unless they were asymptomatic for at least 8 weeks, who do not need glucocorticoid and mannitol to reduce intracranial pressure, and at least one measurable lesion other than brain metastasis, and more than 4 weeks between brain radiotherapy and the last radiotherapy.
Additional exclusion criteria were severe cardiopulmonary, hepatorenal dysfunction, severe or uncontrolled infection, unhealed wound, traumatic bone fracture, hypertension and uncontrolled by antihypertensive drugs, grade >1 myocardial ischemia or myocardial infarction, grade ≥1 arrhythmia (QT prolongation ≥440 ms) or cardiac dysfunction; coagulation disorders, evidence of bleeding diathesis or gastrointestinal bleeding tendency; arteriovenous thrombotic events within 6 months; history of other malignancies within 5 years except basal cell carcinoma of skin and carcinoma in situ of uterine cervix.
The relevant institutional review board or ethics committee of Fudan University Shanghai Cancer Center approved the study, which was conducted in accordance with the Declaration of Helsinki. All patients provided written informed consent.
Study design and treatment
The NAN trial was an open-label, randomized, phase 2 trial evaluating the safety and efficacy of adding apatinib to chemotherapy in patients with advanced TNBC with failed first/second-line treatment in the metastatic setting. A total of 66 patients were randomly assigned, in a 1:1 ratio, to receive vinorelbine or vinorelbine plus apatinib. Stratification was by visceral metastasis (yes vs. no) and previous treatment (first-line vs. second-line). Vinorelbine monotherapy was administered as a once-per-week intravenous infusion of 25 mg/m2 on days 1, 8, and 15 of each 28-day treatment cycle. In vinorelbine plus apatinib group, vinorelbine in administered intravenously at a dose of 20 mg/m2 on day 7, 14, and 21 of each 28-day treatment cycle. Apatinib 250 mg was administered orally once daily at a fixed time each day about 30 min after a meal, with treatment on days 1 to 5 of weeks 1, 2, and 3 within each 28-day cycle (if tolerable, the second cycle started with 500 mg per day).
Dose modifications were regulated by protocol-specified toxicity criteria. Tumor assessment of evaluable lesions was performed by computed tomography scanning or magnetic resonance imaging at baseline (4 weeks before treatment), every two cycles (every 6 weeks ± 3 days) during treatment, and ECOG was performed at the same time. According to the RECIST 1.1 criteria63, patients with CR, PR and SD continued treatments until disease progression, development of unacceptable toxicity, or withdrawal of consent.
The primary endpoint was progression-free survival (PFS), which was defined as the time from randomization to objective tumor progression (according to RECIST 1.1) or death from any cause, whichever occurred first. Secondary end points included overall survival (OS), overall response rate (ORR) and safety. OS was defined as the time from randomization to last follow up or death from any cause. ORR was defined as the proportion of patients whose best objective response was confirmed complete or partial response before disease progression. Safety and adverse events including Hematological and nonhematological toxicity were graded and assessed with the use of the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE version 4.0).
Statistical analysis
According to the available data on phase III trials enrolling TNBC patients, the PFS with vinorelbine plus apatinib would have been increased from 2.5 months of vinorelbine monotherapy to 5.5 months (24 months enrollment duration, 12 months follow-up duration after enrollment), with 80% power at a 5% significance level. A sample of 66 evaluable patients were to be enrolled assuming <10% patient discontinuation rate.
Statistical analyses were performed using SPSS 23.0 for Windows (IBM, Armonk, NY, USA). The means were calculated for age variable, and percentages were calculated for clinicopathological variables. Survival end points were constructed by the Kaplan‐Meier method, and the difference was detected by log‐rank test. Subgroup analyses were performed using a stratified Cox proportional-hazards model with two-sided 95% confidence intervals. Efficacy data were analyzed on an intention-to-treat basis. Categorical variables analysis was performed using the Pearson test or the Fisher exact test. Safety was assessed in all patients who received at least one dose of the trial treatment. The grade of adverse event during trial treatment was reported. All statistical tests were two‐sided., differences were considered statistically significant if P < 0.05.
This study is registered with ClinicalTrials.gov Identifier: NCT03254654, August 17, 2017. Disclosures provided by the authors and data availability statement (if applicable) are available upon request by contact with the corresponding author.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
Siegel, R. L., Miller, K. D., Fuchs, H. E. & Jemal, A. Cancer Statistics, 2021. CA: Cancer J. Cin. 71, 7–33 (2021).
Ferlay, J. et al. Cancer statistics for the year 2020: an overview. Int. J. Cancer https://doi.org/10.1002/ijc.33588 (2021).
Waks, A. G. & Winer, E. P. Breast cancer treatment: a review. JAMA 321, 288–300 (2019).
Dent, R. et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin. Cancer Res.: Off. J. Am. Assoc. Cancer Res. 13, 4429–4434 (2007).
Bianchini, G., Balko, J. M., Mayer, I. A., Sanders, M. E. & Gianni, L. Triple-negative breast cancer: challenges and opportunities of a heterogeneous disease. Nat. Rev. Clin. Oncol. 13, 674–690 (2016).
Yardley, D. A. et al. nab-Paclitaxel plus carboplatin or gemcitabine versus gemcitabine plus carboplatin as first-line treatment of patients with triple-negative metastatic breast cancer: results from the tnAcity trial. Ann. Oncol.: Off. J. Eur. Soc. Med. Oncol. 29, 1763–1770 (2018).
Gaynor, N., Crown, J. & Collins, D. M. Immune checkpoint inhibitors: key trials and an emerging role in breast cancer. Semin. Cancer Biol. https://doi.org/10.1016/j.semcancer.2020.06.016 (2020).
Molinero, L. et al. Tumor immune microenvironment and genomic evolution in a patient with metastatic triple negative breast cancer and a complete response to atezolizumab. J. Immunother. cancer 7, 274 (2019).
Tomioka, N. et al. The therapeutic candidate for immune checkpoint inhibitors elucidated by the status of tumor-infiltrating lymphocytes (TILs) and programmed death ligand 1 (PD-L1) expression in triple negative breast cancer (TNBC). Breast Cancer 25, 34–42 (2018).
Barchiesi, G. et al. Emerging role of PARP inhibitors in metastatic triple negative breast cancer. current scenario and future perspectives. Front. Oncol. 11, 769280 (2021).
Damaskos, C. et al. Triple-negative breast cancer: the progress of targeted therapies and future tendencies. Anticancer Res. 39, 5285–5296 (2019).
Müller, V. et al. First-line bevacizumab-containing therapy for HER2-negative locally advanced/metastatic breast cancer: real-world experience from >2000 patients treated in the multicentre AVANTI study. Breast 60, 70–77 (2021).
Schneider, B. P. et al. Prognostic and predictive value of tumor vascular endothelial growth factor gene amplification in metastatic breast cancer treated with paclitaxel with and without bevacizumab; results from ECOG 2100 trial. Clin. Cancer Res.: Off. J. Am. Assoc. Cancer Res. 19, 1281–1289 (2013).
Robert, N. J. et al. RIBBON-1: randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative, locally recurrent or metastatic breast cancer. J. Clin. Oncol.: Off. J. Am. Soc. Clin. Oncol. 29, 1252–1260 (2011).
Miles, D. W. et al. Phase III study of bevacizumab plus docetaxel compared with placebo plus docetaxel for the first-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J. Clin. Oncol.: Off. J. Am. Soc. Clin. Oncol. 28, 3239–3247 (2010).
Gray, R., Bhattacharya, S., Bowden, C., Miller, K. & Comis, R. L. Independent review of E2100: a phase III trial of bevacizumab plus paclitaxel versus paclitaxel in women with metastatic breast cancer. J. Clin. Oncol.: Off. J. Am. Soc. Clin. Oncol. 27, 4966–4972 (2009).
Miller, K. et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N. Engl. J. Med. 357, 2666–2676 (2007).
Mavroudis, D. et al. Randomized phase III trial comparing docetaxel plus epirubicin versus docetaxel plus capecitabine as first-line treatment in women with advanced breast cancer. Ann. Oncol.: Off. J. Eur. Soc. Med. Oncol. 21, 48–54 (2010).
Brufsky, A. M. et al. RIBBON-2: a randomized, double-blind, placebo-controlled, phase III trial evaluating the efficacy and safety of bevacizumab in combination with chemotherapy for second-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J. Clin. Oncol.: Off. J. Am. Soc. Clin. Oncol. 29, 4286–4293 (2011).
Rugo, H. S. et al. Randomized phase III trial of paclitaxel once per week compared with nanoparticle albumin-bound nab-paclitaxel once per week or ixabepilone with bevacizumab as first-line chemotherapy for locally recurrent or metastatic breast cancer: CALGB 40502/NCCTG N063H (Alliance). J. Clin. Oncol.: Off. J. Am. Soc. Clin. Oncol. 33, 2361–2369 (2015).
Rossari, J. R. et al. Bevacizumab and breast cancer: a meta-analysis of first-line phase III studies and a critical reappraisal of available evidence. J. Oncol. 2012, 417673 (2012).
Fathi Maroufi, N. et al. Therapeutic potentials of apatinib in cancer treatment: possible mechanisms and clinical relevance. Life Sci. 241, 117106 (2020).
Li, J. et al. Randomized, double-blind, placebo-controlled phase III trial of apatinib in patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. J. Clin. Oncol.: Off. J. Am. Soc. Clin. Oncol. 34, 1448–1454 (2016).
Gao, Z., Shi, M., Wang, Y., Chen, J. & Ou, Y. Apatinib enhanced anti-tumor activity of cisplatin on triple-negative breast cancer through inhibition of VEGFR-2. Pathol. Res. Pract. 215, 152422 (2019).
Lan, C. Y. et al. Apatinib combined with oral etoposide in patients with platinum-resistant or platinum-refractory ovarian cancer (AEROC): a phase 2, single-arm, prospective study. Lancet Oncol. 19, 1239–1246 (2018).
Hu, X. et al. Multicenter phase II study of apatinib, a novel VEGFR inhibitor in heavily pretreated patients with metastatic triple-negative breast cancer. Int. J. Cancer 135, 1961–1969 (2014).
Cardoso, F. et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann. Oncol.: Off. J. Eur. Soc. Med. Oncol. 31, 1623–1649 (2020).
Zhang, J. et al. A phase II trial of biweekly vinorelbine and oxaliplatin in second- or third-line metastatic triple-negative breast cancer. Cancer Biol. Ther. 16, 225–232 (2015).
Madu, C. O., Wang, S., Madu, C. O. & Lu, Y. Angiogenesis in breast cancer progression, diagnosis, and treatment. J. Cancer 11, 4474–4494 (2020).
Vasudev, N. S. & Reynolds, A. R. Anti-angiogenic therapy for cancer: current progress, unresolved questions and future directions. Angiogenesis 17, 471–494 (2014).
Tian, Z., Niu, X. & Yao, W. Efficacy and response biomarkers of apatinib in the treatment of malignancies in China: a review. Front. Oncol. 11, 749083 (2021).
Ma, S. et al. The role of tumor microenvironment in resistance to anti-angiogenic therapy. F1000Research 7, 326 (2018).
Liu, J. et al. Biomarkers of response to camrelizumab combined with apatinib: an analysis from a phase II trial in advanced triple-negative breast cancer patients. Breast Cancer Res. Treat. 186, 687–697 (2021).
Tang, D. et al. Apatinib-induced NF-κB inactivation sensitizes triple-negative breast cancer cells to doxorubicin. Am. J. Transl. Res. 12, 3741–3753 (2020).
Liu, H. et al. Giant fungated locally advanced breast carcinoma responded to hypofractionated radiotherapy combined with apatinib: a case report and literature review. Cancer Manag. Res. 13, 605–611 (2021).
Li, Y. H. et al. Comparison of apatinib and capecitabine (Xeloda) with capecitabine (Xeloda) in advanced triple-negative breast cancer as third-line therapy: a retrospective study. Medicine 97, e12222 (2018).
Zeichner, S. B., Terawaki, H. & Gogineni, K. A review of systemic treatment in metastatic triple-negative breast cancer. Breast Cancer. Basic Clin. Res. 10, 25–36 (2016).
Lorusso, V., Latorre, A. & Giotta, F. Chemotherapy options beyond the first line in HER-negative metastatic breast cancer. J. Oncol. 2020, 9645294 (2020).
Li, J. et al. Adjuvant capecitabine with docetaxel and cyclophosphamide plus epirubicin for triple-negative breast cancer (CBCSG010): an open-label, randomized, multicenter, phase III trial. J. Clin. Oncol.: Off. J. Am. Soc. Clin. Oncol. 38, 1774–1784 (2020).
Mayer, I. A. et al. Randomized phase III postoperative trial of platinum-based chemotherapy versus capecitabine in patients with residual triple-negative breast cancer following neoadjuvant chemotherapy: ECOG-ACRIN EA1131. J. Clin. Oncol.: Off. J. Am. Soc. Clin. Oncol. 39, 2539–2551 (2021).
Park, I. H. et al. Randomized open label phase III trial of irinotecan plus capecitabine versus capecitabine monotherapy in patients with metastatic breast cancer previously treated with anthracycline and taxane: PROCEED Trial (KCSG BR 11-01). Cancer Res. Treat. 51, 43–52 (2019).
Roché, H. & Vahdat, L. T. Treatment of metastatic breast cancer: second line and beyond. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 22, 1000–1010 (2011).
Capasso, A. Vinorelbine in cancer therapy. Curr. Drug Targets 13, 1065–1071 (2012).
Dranitsaris, G., Gluck, S., Faria, C., Cox, D. & Rugo, H. Comparative effectiveness analysis of monotherapy with cytotoxic agents in triple-negative metastatic breast cancer in a community setting. Clin. Ther. 37, 134–144 (2015).
Wang, J. et al. Efficacy and safety of vinorelbine plus cisplatin vs. gemcitabine plus cisplatin for treatment of metastatic triple-negative breast cancer after failure with anthracyclines and taxanes. Med. Sci. Monit.: Int. Med. J. Exp. Clin. Res. 23, 4657–4664 (2017).
Rodler, E. T. et al. Phase I study of veliparib (ABT-888) combined with cisplatin and vinorelbine in advanced triple-negative breast cancer and/or BRCA mutation-associated breast cancer. Clin. Cancer Res.: Off. J. Am. Assoc. Cancer Res. 22, 2855–2864 (2016).
Pallis, A. G. et al. A multicenter randomized phase III trial of vinorelbine/gemcitabine doublet versus capecitabine monotherapy in anthracycline- and taxane-pretreated women with metastatic breast cancer. Ann. Oncol.: Off. J. Eur. Soc. Med. Oncol. 23, 1164–1169 (2012).
Palmieri, C. et al. A randomized feasibility study of docetaxel versus vinorelbine in advanced breast cancer. Oncologist 17, 1429–e1447 (2012).
Aapro, M. et al. Randomized phase II study evaluating weekly oral vinorelbine versus weekly paclitaxel in estrogen receptor-positive, HER2-negative patients with advanced breast cancer (NorBreast-231 trial). Breast 45, 7–14 (2019).
Luu, T. et al. Phase I/II trial of vinorelbine and sorafenib in metastatic breast cancer. Clin. Breast Cancer 14, 94–100 (2014).
Cortes, J. et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet 396, 1817–1828 (2020).
Emens, L. A. et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer: biomarker evaluation of the IMpassion130 study. J. Natl Cancer Inst. 113, 1005–1016 (2021).
Emens, L. A. et al. Long-term clinical outcomes and biomarker analyses of atezolizumab therapy for patients with metastatic triple-negative breast cancer: a phase 1 study. JAMA Oncol. 5, 74–82 (2019).
Litton, J. K. et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N. Engl. J. Med. 379, 753–763 (2018).
Robson, M. et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N. Engl. J. Med. 377, 523–533 (2017).
Robson, M. E. et al. OlympiAD final overall survival and tolerability results: olaparib versus chemotherapy treatment of physician’s choice in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer. Ann. Oncol.: Off. J. Eur. Soc. Med. Oncol. 30, 558–566 (2019).
Adams, E., Wildiers, H., Neven, P. & Punie, K. Sacituzumab govitecan and trastuzumab deruxtecan: two new antibody-drug conjugates in the breast cancer treatment landscape. ESMO Open 6, 100204 (2021).
Bardia, A. et al. Sacituzumab Govitecan-hziy in refractory metastatic triple-negative breast cancer. N. Engl. J. Med. 380, 741–751 (2019).
Ahmed, F. S. et al. PD-L1 protein expression on both tumor cells and macrophages are associated with response to neoadjuvant durvalumab with chemotherapy in triple-negative breast cancer. Clin. Cancer Res.: Off. J. Am. Assoc. Cancer Res. 26, 5456–5461 (2020).
Jiang, Y. Z. et al. Molecular subtyping and genomic profiling expand precision medicine in refractory metastatic triple-negative breast cancer: the FUTURE trial. Cell Res. 31, 178–186 (2021).
Voorwerk, L. et al. Immune induction strategies in metastatic triple-negative breast cancer to enhance the sensitivity to PD-1 blockade: the TONIC trial. Nat. Med. 25, 920–928 (2019).
Wang, Q., Gao, J., Di, W. & Wu, X. Anti-angiogenesis therapy overcomes the innate resistance to PD-1/PD-L1 blockade in VEGFA-overexpressed mouse tumor models. Cancer Immunol., Immunother. 69, 1781–1799 (2020).
Eisenhauer, E. A. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer 45, 228–247 (2009).
Acknowledgements
We thank Dr. Changming Zhou (Department of Clinical Statistics, Fudan University Shanghai Cancer Center) and Dr. Wentao Shi (Department of Clinical Statistics, Shanghai Ninth People’s Hospital) for analyzing clinical data.
Funding
This study was supported by National Natural Science Foundation of China (grant no. 82072915); the Shanghai Municipal Science and Technology Commission Guidance Project, P.R. China (contract no. 18411967800); research grant from Shanghai Hospital Development Center (grant no. SHDC12018X03); CSCO-ROCHE Cancer Research Fund 2019 (grant no. Y-2019Roche-171); and Chinese Young Breast Experts Research project (grant no. CYBER-2021-001).
Author information
Authors and Affiliations
Contributions
Conception and design: JZ and X-CH; Administrative support: X-CH. Collection and assembly of data: D-DL, Z-hT, B-YW, L-PW, JC; Data analysis and interpretation: D-DL and JZ; Manuscript writing: D-DL and JZ; Final approval of manuscript: All authors; Accountable for all aspects of the work: All authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, DD., Tao, Zh., Wang, BY. et al. Apatinib plus vinorelbine versus vinorelbine for metastatic triple-negative breast cancer who failed first/second-line treatment: the NAN trial. npj Breast Cancer 8, 110 (2022). https://doi.org/10.1038/s41523-022-00462-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41523-022-00462-6
This article is cited by
-
Recent advances in targeted strategies for triple-negative breast cancer
Journal of Hematology & Oncology (2023)
-
Pretreatment lymphocyte-monocyte ratio (LMR) as a superior predictor of short-term progression outcomes in patients with gastric cancer receiving second- and later-line apatinib regimens
Journal of Cancer Research and Clinical Oncology (2023)