Abstract
Observational studies have suggested that HER2 inhibition with trastuzumab may be associated with an increased incidence of intracranial metastatic disease (IMD) due to its ability to prolong survival. We hypothesized that prolonged survival associated with dual-agent HER2 inhibition may be associated with an even higher incidence of IMD. This study pooled estimates of IMD incidence and survival among patients with HER2-positive breast cancer receiving dual- versus single-agent HER2 targeted therapy, as well as trastuzumab versus chemotherapy, observation, or another HER2-targeted agent. We searched PubMed, EMBASE, and CENTRAL from inception to 25 March 2020. We included randomized controlled trials that reported IMD incidence for patients with HER2-positive breast cancer receiving trastuzumab as the experimental or control arm irrespective of disease stage. Among 465 records identified, 19 randomized controlled trials (32,572 patients) were included. Meta-analysis of four studies showed that dual HER2-targeted therapy was associated with improved overall survival (HR 0.76; 95% CI, 0.66–0.87) and progression-free survival (HR 0.77; 95% CI, 0.68–0.87) compared to single HER2-targeted therapy, but the risk of IMD was similar (RR 1.03; 95% CI, 0.83–1.27). Our study challenges the hypothesis that prolonged survival afforded by improved extracranial disease control is associated with increased IMD incidence.
Similar content being viewed by others
Introduction
Intracranial metastatic disease (IMD) is a common and serious complication of breast cancer1, with a median survival of 13.8 months2 and reduced quality of life due to disease symptomatology and treatment toxicity3. Breast cancers expressing human epidermal growth factor receptor 2 (HER2) have a higher propensity to metastasize to the central nervous system (CNS) compared to hormone receptor (HR)-positive/HER2-negative disease subtypes4,5
The anti-HER2-monoclonal antibody trastuzumab has been shown to improve overall survival (OS) for HER2-positive breast cancer patients and has become standard of care6,7. However, case series and cohort studies have reported a higher incidence of IMD among HER2-positive patients treated with trastuzumab for metastatic, unresectable, or recurrent breast cancer8,9,10,11,12,13,14,15,16. Meta-analyses of randomized controlled trials (RCTs) have corroborated such findings in non-metastatic disease, but have not included data accrued in recent years17,18,19,20,21. The increased IMD incidence following treatment with trastuzumab has been attributed to its improvement of OS: trastuzumab controls extracranial disease and prolongs survival until dormant micrometastases within the sanctuary of the CNS are able to proliferate and manifest clinically 22,23.
The effectiveness of HER2 inhibition for HER2-positive breast cancer has motivated the development of novel HER2-targeted agents and trials to determine their efficacy as single agents or in combination with trastuzumab. RCTs have demonstrated the efficacy of several HER2-targeting agents, including anti-HER2 antibodies and conjugates (trastuzumab emtansine and pertuzumab) or HER2-targeted tyrosine kinase inhibitors (TKIs; lapatinib, neratinib, and tucatinib)24,25,26,27,28,29,30,31. Ongoing studies for margetuximab32, pyrotinib33, trastuzumab deruxtecan34, ARX78835, and PRS-34336 may expand upon or improve current options for patients with HER2-positive breast cancer, and illuminate the impact of additional HER2-targeted agents on IMD incidence.
HER2-targeted agents have also garnered interest in the treatment of IMD from HER2-positive breast cancer. Trastuzumab and other HER2-targeted antibodies and conjugates have been associated with a reduced number of intracranial tumors at IMD diagnosis, prolonged post-IMD OS, or intracranial responses, indicating possible intracranial efficacy despite minimal blood-brain barrier penetrance37,38,39. Recent reviews address the landscape of HER2-targeted agents for the management of IMD, and suggest that the introduction of novel agents, greater inclusion of patients with IMD in clinical trials, and increased reporting of intracranial outcomes may all benefit future patients with IMD40,41.
The purpose of this systematic review and meta-analysis was to update existing estimates of the incidence of IMD among patients with HER2+ breast cancer, and to assess the impact of novel HER2-targeted regimens on the development of intracranial metastases. To address this question, we assessed IMD incidence and survival among patients with HER2-positive breast cancer who were treated with dual anti-HER2 therapy versus trastuzumab monotherapy; we also performed meta-analysis of IMD incidence in patients receiving trastuzumab versus chemotherapy, observation, or another HER2-targeted agent.
Methods
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines42.
Search strategy
We searched MEDLINE (via PubMed), EMBASE (via Wiley), and CENTRAL (via Cochrane) on March 25, 2020. We also screened references of eligible articles and reviews, and queried Google Scholar, PubMed, and ClinicalTrials.gov for updated or IMD-specific publications of trials at full-text review. Full search queries are presented in the supplement (Supplementary Tables 1–3).
Study selection
Using a two-step process, we screened abstracts and then full texts of selected records to identify RCTs that reported the incidence of IMD that compared dual HER2-targeted regimens to trastuzumab, trastuzumab to another HER2-targeted therapy, or trastuzumab to standard chemotherapy or observation. Studies were screened in duplicate by two independent reviewers (AE, CH), and Cohen’s κ statistic was calculated for inter-rater reliability at both steps. Disagreements were resolved through discussion. Studies could report IMD incidence overall or as the site of the first recurrence. Trials that did not report IMD incidence were excluded. Gray literature sources were not searched. Conference abstracts were eligible. No date range was applied, but studies were required to be in English. Full inclusion and exclusion criteria are presented in the supplement (Tables S4–5).
Data extraction and quality assessment
The following data were extracted from included studies: trial name, treatment procedures, median follow-up, prior treatments, early (stage I–II) versus advanced (stage III–IV) HER2-positive breast cancer, number of intracranial events, number of recurrence events, OS (hazard ratio) and progression-free survival (PFS, hazard ratio), reported either as disease-free, progression-free, or event-free survival. Outcomes specific to intention-to-treat analyses were preferentially extracted. Data were extracted by a single reviewer due to resource constraints. We performed a quality assessment using the Cochrane Risk of Bias 2 tool (RoB 2) to evaluate risk of bias across five domains (randomization, deviation from intended interventions, missing outcomes, measurement bias, and selection bias) and overall43. We assessed evidence quality using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) framework 44.
Data synthesis and analysis
We performed a meta-analysis using the inverse variance method with random-effects models to produce summary risk ratios (RR) and hazard ratios (HR). We calculated RR using the number of cases of incident IMD divided by the number of patients in the intention-to-treat population per study arm. HR were extracted as adjusted HR when available, otherwise as reported. Missing HR values from one study6 were imputed using the method by Guyot et al.45 from digitized Kaplan–Meier plots and at-risk tables.
To assess heterogeneity, we calculated the Q-statistic for the ratio of observed to within-study variance, τ2 for between-study variance, and I2 for the percentage of observed variance attributable to between-study variance46,47.
We evaluated the incidence of IMD among patients receiving HER2-targeted therapy through two separate comparisons: dual HER2-targeted therapy versus trastuzumab; and trastuzumab versus chemotherapy, observation, or another anti-HER agent. Patients were pooled from treatment arms that received the same chemotherapy plus trastuzumab combination, but in concurrent or sequential order, or for different durations. As subgroup analyses, we estimated summary effects stratifying by disease stage, and in comparisons of trastuzumab monotherapy to control regimens, by the comparator. As sensitivity analyses, we compared summary estimates from fixed- and random-effects models, and omitted studies one at a time as a “leave-out-one” assessment47,48.
We assessed publication bias by examining the funnel plot asymmetry visually and with Egger’s test49. All statistical analysis was conducted using the R programming language (v3.6.1, R Foundation for Statistical Computing)50 and the R package meta51.
Results
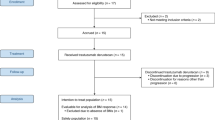
Our search identified 465 unique records, from which we reviewed 65 full-text articles. Eighteen studies6,25,28,29,52,53,54,55,56,57,58,59,60,61,62,63,64,65 reporting on nineteen trials met eligibility criteria (Fig. 1); notably, 31 of 65 studies at full-text screening were excluded due to lack of reported IMD incidence. Cohen’s κ at the abstract (0.64) and full text (0.66) screening indicated substantial agreement between reviewers48. In total, included RCTs involved 32,572 patients with HER2-positive breast cancer. Four studies25,52,53,59 compared dual HER2-targeted therapy to trastuzumab (n = 10,103), seven studies6,55,56,57,58,60,65 compared trastuzumab to chemotherapy or observation (n = 13,752), and nine studies28,29,52,53,54,61,62,63,64 compared trastuzumab to another HER2-targeted agent (n = 13,207). Ten of the eighteen eligible studies involved patients with early stage disease. Median follow-up ranged from 9 months to 11 years from the start of trial therapy. Regular intracranial CT/MRI imaging was reported by only two studies. The characteristics of these trials are presented in Table 1 and Supplementary Table 6.
Search queries were conducted in PubMed, EMBASE, and CENTRAL from their inception to 25 March 2020 for randomized controlled trials investigating trastuzumab that reported incidence of intracranial metastatic disease42.
Dual HER2-targeted therapy was associated with prolonged OS (four studies; HR 0.76; 95% CI, 0.66–0.87; Fig. 2; GRADE high) and PFS (four studies; HR 0.77; 95% CI, 0.68–0.87; Fig. 3; GRADE high) compared to single HER2-targeted therapy with trastuzumab. Heterogeneity in these comparisons was low (I2 = 0% for OS and 11% for PFS). Stratification by disease stage showed dual HER2-targeted therapy was associated with prolonged OS and PFS in both early stage (OS: three studies; HR 0.82; 95% CI, 0.68–0.99; Fig. 2. PFS: three studies; HR 0.82; 95% CI, 0.72–0.94; Fig. 3) and advanced-stage disease (OS: 1 study; HR 0.68; 95% CI, 0.56–0.83; Fig. 2. PFS: 1 study; HR 0.68; 95% CI, 0.58–0.80; Fig. 3).
Hazard ratios for overall survival were extracted from eligible studies and pooled using a random-effects model. Studies here are stratified by disease stage: either early (stage I–II) or advanced (stage III–IV). The size of each box represents the weight of each study in the meta-analysis. The vertical solid line represents the point of equivalence between dual and single HER2 therapy. The vertical dashed and dotted lines represent the points of summary for fixed and random effects models, respectively, and the diamonds represent 95% CI for the summary hazard ratios. Analyses were performed with the R programming language50 and the R package meta51.
Hazard ratios for progression-free survival were extracted from eligible studies and pooled using a random-effects model. Studies here are stratified by disease stage: either early (stage I–II) or advanced (stage III–IV). The size of each box represents the weight of each study in the meta-analysis. The vertical solid line represents the point of equivalence between dual and single HER2 therapy. The vertical dashed and dotted lines represent the points of summary for fixed and random effects models, respectively, and the diamonds represent 95% CI for the summary hazard ratios. Analyses were performed with the R programming language50 and the R package meta51.
The risk of IMD incidence was not different between patients receiving dual versus single HER2-targeted therapy (four studies; RR 1.03; 95% CI, 0.83–1.27; Fig. 4). Heterogeneity in this comparison was low (I2 = 0%). Subgroup analysis revealed no difference between early stage (three studies; RR 1.03; 95% CI, 0.78–1.37; Fig. 4) and advanced-stage disease (one study; RR 1.03; 95% CI, 0.74–1.42; Fig. 4), or if the dual-therapy included lapatinib as the second agent (two studies; RR 1.04; 95% CI, 0.70–1.54; Supplementary Fig. 1) versus pertuzumab (two studies; RR 1.03; 95% CI, 0.83–1.64; Supplementary Fig. 1).
Risk ratios were calculated from the proportion of patients in each study arm who developed the intracranial metastatic disease over the study course. Studies here are stratified by disease stage: either early (stage I–II) or advanced (stage III–IV). The size of each box represents the weight of each study in the meta-analysis. The vertical solid line represents the point of equivalence between dual and single HER2 therapy. The vertical dashed and dotted lines represent the points of summary for fixed and random effects models, respectively, and the diamonds represent 95% CI for the summary relative risks. Analyses were performed with the R programming language50 and the R package meta51.
Patients receiving trastuzumab did not show an increased incidence of IMD compared to another HER2-targeted therapy (nine studies; RR 1.15; 95% CI 0.88–1.50), observation (two studies; RR 1.12; 95% CI 0.78–1.60), or chemotherapy (five studies; RR 1.32; 95% CI, 0.88–1.97) (Fig. 5). Heterogeneity in this comparison was moderate overall (I2 = 37%), and higher but still moderate in the chemotherapy (I2 = 57%) and anti-HER2 agent (I2 = 38%) comparator subgroups. The summary estimate for IMD incidence from pooling the seven studies of trastuzumab monotherapy versus chemotherapy or observation comparators was RR 1.27 (95% CI, 0.95–1.70) (Supplementary Fig. 2). Subgroup analysis of studies of early stage disease showed no difference in IMD incidence (RR 1.01; 95% CI, 0.81–1.26; nine studies; Supplementary Fig. 3) between trastuzumab monotherapy versus chemotherapy, observation, or another HER2-targeted agent. Subgroup analysis of studies of advanced-stage disease showed significantly increased IMD incidence (RR 1.53, 95% CI, 1.19–1.97; seven studies; Supplementary Fig. 4) with trastuzumab monotherapy versus chemotherapy or another HER2-targeted agent.
Risk ratios were calculated from the proportion of patients in each study arm who developed the intracranial metastatic disease over the study course. Studies here are stratified by comparator regimen: either chemotherapy, observation, or another HER2-targeted agent. The size of each box represents the weight of each study in the meta-analysis. The vertical solid line represents the point of equivalence between trastuzumab and comparators. The vertical dashed and dotted lines represent the points of summary for fixed and random effects models, respectively, and the diamonds represent 95% CI for the summary relative risks. Analyses were performed with the R programming language50 and the R package meta51.
The overall risk of bias was low in 6/18 (33%) and moderate in 12/18 (66%) included RCTs (Fig. S5). Summary plots for risk of bias showed low to moderate risk for the meta-analyses in this study (Supplementary Fig. 6). Assessment of funnel plots did not indicate publication bias, although these assessments were underpowered in all but one case (Supplementary Figs. 7–11). Sensitivity analysis showed that the findings were robust: comparison of random- and fixed-effects estimates did not render any significant summary estimates insignificant, and iterative omission of each study in the “leave-out-one” analyses did not significantly perturb summary estimates, including one study with follow-up <1 year29. GRADE certainty level was “high” for summary estimates (Table 2).
Discussion
Our study found prolonged OS and PFS without a significant difference in IMD incidence, with the addition of lapatinib or pertuzumab to trastuzumab for patients with HER2-positive breast cancer. Although there is mixed evidence for the ability of lapatinib to penetrate the blood-brain barrier66,67,68, our pooled analysis of two studies failed to show a difference in IMD incidence between lapatinib plus trastuzumab versus trastuzumab alone. While our study was not designed to assess this comparison, future reporting may clarify a role for HER2-targeted TKIs in IMD prevention. Of note, several high-profile trials (HER2CLIMB30, DESTINY34, TH3RESA69, EMILIA24, MARIANNE70) were captured in the literature search but did not meet inclusion criteria because they either have not yet reported IMD incidence, or featured absent or ineligible comparators.
Our study did not find a significant difference in IMD incidence between patients receiving dual anti-HER2 therapy versus trastuzumab alone. We also did not find a difference comparing trastuzumab with chemotherapy or observation, but this is in contradistinction to previous meta-analyses of RCTs17,18,19,20,21. One possible explanation for this difference is that our meta-analysis for this outcome involved more patients and longer follow-up for events to accrue. Further, previous meta-analyses included only studies of early stage disease, but our subgroup analysis of this population did not show a significant difference in IMD incidence between trastuzumab monotherapy and chemotherapy or observation (HR 1.01; 95% CI, 0.81–1.26; p = 0.92; Supplementary Fig. 3). Conversely, our subgroup analysis of advanced-stage disease showed an association between trastuzumab monotherapy and increased incidence of IMD, compared to chemotherapy or another HER2-targeted agent (HR 1.53; 95% CI, 1.19–1.97; p = 0.001; Supplementary Fig. 4).
These subgroup findings suggest that IMD may be more likely among patients with advanced-stage HER2+ breast cancer who receive trastuzumab monotherapy as compared to chemotherapy or another HER2-targeted agent. These findings also suggest that the impact of dual- versus single-agent HER2-therapy on IMD incidence could be different for patients with advanced- versus early stage disease. Our study did not detect such a difference (p = 0.97; Fig. 4), but this could be due to the small number of studies (advanced: n = 1, early: n = 3) in this comparison. Our subgroup analysis of early stage disease did not show a difference in IMD incidence between patients receiving trastuzumab monotherapy versus chemotherapy or observation. However, this does not rule out the possibility that trastuzumab monotherapy influences IMD incidence for these patients, as the rarity of IMD in these studies renders this estimate susceptible to confounding.
GRADE certainty level was high for summary estimates, although some indirectness may be present in the estimates due to pooling studies of early- and advanced-stage disease. As well, the magnitudes of summary effect sizes were modest.
Our study has several limitations. First, our study did not distinguish between the incidence of IMD overall versus the incidence of IMD as the first site of recurrence. This may have biased our results towards a lower estimate of overall IMD incidence, although one would expect this bias to impact both experimental and control arms similarly. The impact of HER2-targeted agents on IMD incidence overall versus as the first site of recurrence remains underexplored71. Second, patients were pooled from treatment arms that received the same chemotherapy plus trastuzumab combination, but in concurrent or sequential order, or for different durations. This was necessary because there were few studies available for pooling. However, omitting these studies one at a time in the sensitivity analysis did not change our conclusions. Third, our study did not take into account the duration of HER2-targeted regimens, which is a potential confounder, although data from the HERA study suggest no difference in OS or IMD incidence between 1-year and 2-year trastuzumab treatment arms55. Fourth, IMD was not the main outcome of most RCTs, and RR was reported instead of HR. For patients with non-metastatic breast cancer, the occurrence of IMD may take years to develop, and differences in time-to-event comparisons may be obscured by comparing RR across studies with different follow-up durations. Future studies are needed to establish whether HER2-targeted therapy delays rather than prevent IMD. Fifth, blinding was infrequent in the included studies, which could have resulted in an overestimation of survival benefit for patients receiving the study regimen in included studies due to performance bias. Sixth, regular intracranial imaging was reported by only two studies, which could have resulted in an underestimation of a difference in IMD incidence between groups. Seventh, this study was unable to address the impact of the therapy lines on outcomes due to too few studies. Finally, our results may have been impacted by the biases associated with RCTs. Attrition bias may have led to underestimates of IMD incidence following HER2-targeted therapy, although this is mitigated by the majority of included studies reporting analyses based on intention-to-treat populations25,28,52,54,55,57,58,59,62,72,73,74,75,76. Although most studies excluded at full-text review did not report IMD incidence despite evaluating the incidence of other safety outcomes in patients receiving HER2-targeted agents, we were unable to demonstrate publication bias with the small number of studies included.
While other meta-analyses have assessed survival and safety between dual- and single-agent HER2-targeted regimens77,78,79, our study compares IMD incidence in these groups and provides the most recent update on the risk of IMD incidence following trastuzumab monotherapy. Our data suggest prolonged survival may not be associated with increased risk of IMD incidence when comparing dual to single HER2-targeted therapy, and when comparing trastuzumab to chemotherapy and observation in early stage disease. In an effort to maximize the knowledge gained from randomized treatment allocation, follow-up studies of these trials focusing on the incidence of IMD should be conducted. In addition, the incidence of IMD should be prospectively collected in future studies that involve patients who are at high risk of central nervous system metastases.
Conclusions
Dual-agent HER2-targeted therapy for eligible patients with breast cancer is associated with prolonged survival without increased risk of IMD compared to trastuzumab. Together, these findings suggest dual HER2-targeted therapy is associated with decreased IMD risk per unit time. The incidence of IMD among patients receiving trastuzumab was not greater than those who received other HER2-targeted agents, chemotherapy, or observation in the setting of early stage disease. Future trials should monitor and report IMD incidence to assess whether the novel and existing systemic therapies may further impact IMD epidemiology across breast cancer subtypes.
Data availability
The authors declare that the data supporting the findings of this study are available within the paper and its supplementary information files.
Code availability
The code developed during this study is available upon reasonable request. Analyses were performed using the R programming language version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria).
References
Barnholtz-Sloan, J. S. et al. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J. Clin. Oncol. 22, 2865–2872 (2004).
Sperduto, P. W. et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J. Clin. Oncol. 30, 419–425 (2012).
Peters, S., Bexelius, C., Munk, V. & Leighl, N. The impact of brain metastasis on quality of life, resource utilization and survival in patients with non-small-cell lung cancer. Cancer Treat. Rev. 45, 139–162 (2016).
Martin, A. M. et al. Brain metastases in newly diagnosed breast cancer: a population-based study. JAMA Oncol. 3, 1069–1077 (2017).
Buonomo, O. C. et al. New insights into the metastatic behavior after breast cancer surgery, according to well-established clinicopathological variables and molecular subtypes. PLoS ONE https://doi.org/10.1371/journal.pone.0184680 (2017).
Slamon, D. J. et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med. 344, 783–792 (2001).
Piccart-Gebhart, M. J. et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N. Engl. J. Med. 353, 1659–1672 (2005).
Clayton, A. J. et al. Incidence of cerebral metastases in patients treated with trastuzumab for metastatic breast cancer. Br. J. Cancer 91, 639–643 (2004).
Bendell, J. C. et al. Central nervous system metastases in women who receive trastuzumab-based therapy for metastatic breast carcinoma. Cancer 97, 2972–2977 (2003).
Witzel, I. et al. Management of patients with brain metastases receiving trastuzumab treatment for metastatic breast cancer. Onkologie 34, 304–308 (2011).
Park, Y. H. et al. Trastuzumab treatment improves brain metastasis outcomes through control and durable prolongation of systemic extracranial disease in HER2-overexpressing breast cancer patients. Br. J. Cancer 100, 894–900 (2009).
Montagna, E. et al. Central nervous system metastases in a cohort of metastatic breast cancer patients treated with trastuzumab. Cancer Chemother. Pharmacol. 63, 275–280 (2009).
Gori, S. et al. Central nervous system metastases in HER-2-positive metastatic breast cancer patients treated with trastuzumab: incidence, survival, and risk factors. Oncologist 12, 766–773 (2007).
Heinrich B, Brudler O, Siekiera W, et al. Development of brain metastasis in metastatic breast cancer responding to treatment with trastuzumab. Proc Am Soc Clin Oncol 22, abstr. 147 (2003).
Shmueli, E., Wigler, N. & Inbar, M. Central nervous system progression among patients with metastatic breast cancer responding to trastuzumab treatment. Eur. J. Cancer 40, 379–382 (2004).
Yau, T. et al. Incidence, pattern and timing of brain metastases among patients with advanced breast cancer treated with trastuzumab. Acta Oncologica 45, 196–201 (2006).
Olson, E. M. et al. Incidence and risk of central nervous system metastases as site of first recurrence in patients with HER2-positive breast cancer treated with adjuvant trastuzumab. Ann. Oncol. 24, 1526–1533 (2013).
Moja, L. et al. Trastuzumab containing regimens for early breast cancer. Cochrane Database Syst. Rev. CD006243 (2012).
Yin, W., Jiang, Y., Shen, Z., Shao, Z. & Lu, J. Trastuzumab in the adjuvant treatment of HER2-positive early breast cancer patients: a meta-analysis of published randomized controlled trials. PLoS ONE 6, e21030 (2011).
Viani, G. A., Afonso, S. L., Stefano, E. J., De Fendi, L. I. & Soares, F. V. Adjuvant trastuzumab in the treatment of her-2-positive early breast cancer: a meta-analysis of published randomized trials. BMC Cancer 7, 153 (2007).
Bria, E. et al. Cardiotoxicity and incidence of brain metastases after adjuvant trastuzumab for early breast cancer: the dark side of the moon? A meta-analysis of the randomized trials. Breast Cancer Res. Treat. 109, 231–239 (2008).
Palmieri, D., Chambers, A. F., Felding-Habermann, B., Huang, S. & Steeg, P. S. The biology of metastasis to a sanctuary site. Clin. Cancer Res. 13, 1656–1662 (2007).
Heyn, C. et al. In vivo MRI of cancer cell fate at the single-cell level in a mouse model of breast cancer metastasis to the brain. Magn. Reson. Med. 56, 1001–1010 (2006).
Verma, S. et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N. Engl. J. Med. 367, 1783–1791 (2012).
von Minckwitz, G. et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N. Engl. J. Med. 377, 122–131 (2017).
Swain, S. M. et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N. Engl. J. Med. 372, 724–734 (2015).
Geyer, C. E. et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N. Engl. J. Med. 355, 2733–2743 (2006).
Awada, A. et al. Neratinib plus paclitaxel vs trastuzumab plus paclitaxel in previously untreated metastatic ERBB2-positive breast cancer: the NEfERT-T randomized clinical trial. JAMA Oncol. 2, 1557–1564 (2016).
Harbeck, N. et al. Afatinib plus vinorelbine versus trastuzumab plus vinorelbine in patients with HER2-overexpressing metastatic breast cancer who had progressed on one previous trastuzumab treatment (LUX-Breast 1): an open-label, randomised, phase 3 trial. Lancet Oncol. 17, 357–366 (2016).
Murthy, R. K. et al. Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer. N. Engl. J. Med. 382, 597–609 (2020).
Baselga, J. et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet 379, 633–640 (2012).
Rugo, H. S. et al. (American Society of Clinical Oncology, 2019).
Xu, B. et al. A randomized phase II trial of pyrotinib plus capecitabine versus lapatinib plus capecitabine in patients with HER2-positive metastatic breast cancer previously treated with taxanes, anthracyclines and/or trastuzumab. Cancer Research Conference, San Antonio Breast Cancer Symposium, SABCS 2017. United States. 2078 (2014 Supplement 2011) (no pagination), https://doi.org/10.1158/15387445.SABCS17-PD3-08 (2018).
Modi, S. et al. Trastuzumab Deruxtecan in previously treated HER2-positive breast cancer. N. Engl. J. Med. 382, 610–621 (2020).
Barok, M. et al. ARX788, a novel anti-HER2 antibody-drug conjugate, shows anti-tumor effects in preclinical models of trastuzumab emtansine-resistant HER2-positive breast cancer and gastric cancer. Cancer Lett. 473, 156–163 (2020).
Hinner, M. J. et al. Tumor-localized costimulatory T-cell engagement by the 4-1BB/HER2 bispecific antibody-anticalin fusion PRS-343. Clin. Cancer Res. 25, 5878–5889 (2019).
Laakmann, E. et al. Radiological Patterns of Brain Metastases in Breast Cancer Patients: A Subproject of the German Brain Metastases in Breast Cancer (BMBC) Registry. Int. J. Mol. Sci. 17, 23 (2016).
Mounsey, L. A. et al. Changing natural history of HER2-positive breast cancer metastatic to the brain in the era of new targeted therapies. Clin. Breast Cancer 18, 29–37 (2018).
Okines, A. et al. Development and responses of brain metastases during treatment with trastuzumab emtansine (T-DM1) for HER2 positive advanced breast cancer: a single institution experience. Breast J. 24, 253–259 (2018).
Soffietti, R., Ahluwalia, M., Lin, N. & Rudà, R. Management of brain metastases according to molecular subtypes. Nat. Rev. Neurol. 16, 557–574 (2020).
Zimmer, A. S., Van Swearingen, A. E. D. & Anders, C. K. HER2-positive breast cancer brain metastasis: A new and exciting landscape. Cancer Rep. e1274 (2020). https://doi.org/10.1002/cnr2.1274.
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G. & Group, P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J. Clin. Epidemiol. 62, 1006–1012 (2009).
Sterne, J. A. C. et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 366, l4898 (2019).
Guyatt, G. H. et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336, 924–926 (2008).
Guyot, P., Ades, A. E., Ouwens, M. J. & Welton, N. J. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med. Res. Methodol. 12, 9 (2012).
Higgins, J. P., Thompson, S. G., Deeks, J. J. & Altman, D. G. Measuring inconsistency in meta-analyses. BMJ 327, 557–560 (2003).
Borenstein, M., Hedges, L. V., Higgins, J. P. & Rothstein, H. R. Introduction to Meta-analysis. (John Wiley & Sons, 2011).
Higgins, J. P. et al. Cochrane Handbook for Systematic Reviews of Interventions. (John Wiley & Sons, 2019).
Egger, M., Davey Smith, G., Schneider, M. & Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634 (1997).
Team, R. C. R: a language and environment for statistical computing. https://www.R-project.org/ (2019).
Schwarzer, G. meta: an R package for meta-analysis. R News 7, 40–45 (2007).
Huober, J. et al. Survival outcomes of the NeoALTTO study (BIG 1-06): updated results of a randomised multicenter phase III neoadjuvant clinical trial in patients with HER2-positive primary breast cancer. Eur. J. Cancer 118, 169–177 (2019).
Piccart-Gebhart, M. et al. Adjuvant lapatinib and trastuzumab for early human epidermal growth factor receptor 2-positive breast cancer: results from the randomized phase III adjuvant lapatinib and/or trastuzumab treatment optimization trial. J. Clin. Oncol. 34, 1034–1042 (2016).
Takano, T. et al. A randomized phase II trial of trastuzumab plus capecitabine versus lapatinib plus capecitabine in patients with HER2-positive metastatic breast cancer previously treated with trastuzumab and taxanes: WJOG6110B/ELTOP. Breast 40, 67–75 (2018).
Cameron, D. et al. 11 years’ follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin Adjuvant (HERA) trial. Lancet 389, 1195–1205 (2017).
Perez, E. A. et al. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J. Clin. Oncol. 32, 3744–3752 (2014).
Chan, A. et al. Central nervous system as first site of relapse in patients with HER2 positive early breast cancer treated in the BCIRG-006 trial. Cancer Research Conference, 2018 San Antonio Breast Cancer Symposium. United States. 2079 (2014 Supplement 2011) (no pagination), https://doi.org/10.1158/15387445.SABCS18-PD3-12 (2019).
Spielmann, M. et al. Trastuzumab for patients with axillary-node-positive breast cancer: results of the FNCLCC-PACS 04 trial. J. Clin. Oncol. 27, 6129–6134 (2009).
Swain, S. M. et al. Incidence of central nervous system metastases in patients with HER2-positive metastatic breast cancer treated with pertuzumab, trastuzumab, and docetaxel: results from the randomized phase III study CLEOPATRA. Ann. Oncol. 25, 1116–1121 (2014).
Joensuu, H. et al. Fluorouracil, epirubicin, and cyclophosphamide with either docetaxel or vinorelbine, with or without trastuzumab, as adjuvant treatments of breast cancer: final results of the FinHer Trial. J. Clin. Oncol. 27, 5685–5692 (2009).
Pivot, X. et al. CEREBEL (EGF111438): A Phase III, Randomized, Open-Label Study of Lapatinib Plus Capecitabine Versus Trastuzumab Plus Capecitabine in Patients With Human Epidermal Growth Factor Receptor 2-Positive Metastatic Breast Cancer. J. Clin. Oncol. 33, 1564–1573 (2015).
von Minckwitz, G. et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N. Engl. J. Med. 380, 617–628 (2019).
Untch, M. et al. Survival Analysis After Neoadjuvant Chemotherapy With Trastuzumab or Lapatinib in Patients With Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer in the GeparQuinto (G5) Study (GBG 44). J. Clin. Oncol. 36, 1308–1316 (2018).
Gelmon, K. A. et al. Lapatinib or trastuzumab plus taxane therapy for human epidermal growth factor receptor 2-positive advanced breast cancer: final results of NCIC CTG MA.31. J. Clin. Oncol. 33, 1574–1583 (2015).
von Minckwitz, G. et al. Trastuzumab beyond progression in human epidermal growth factor receptor 2-positive advanced breast cancer: a german breast group 26/breast international group 03-05 study. J. Clin. Oncol. 27, 1999–2006 (2009).
Saleem, A. et al. Lapatinib access into normal brain and brain metastases in patients with Her-2 overexpressing breast cancer. EJNMMI Res. 5, 30 (2015).
Cameron, D. et al. A phase III randomized comparison of lapatinib plus capecitabine versus capecitabine alone in women with advanced breast cancer that has progressed on trastuzumab: updated efficacy and biomarker analyses. Breast Cancer Res. Treat. 112, 533–543 (2008).
Taskar, K. S. et al. Lapatinib distribution in HER2 overexpressing experimental brain metastases of breast cancer. Pharm. Res. 29, 770–781 (2012).
Krop, I. E. et al. Trastuzumab emtansine versus treatment of physician’s choice for pretreated HER2-positive advanced breast cancer (TH3RESA): a randomised, open-label, phase 3 trial. Lancet Oncol. 15, 689–699 (2014).
Perez, E. A. et al. Trastuzumab emtansine with or without pertuzumab versus trastuzumab plus taxane for human epidermal growth factor receptor 2-positive, advanced breast cancer: primary results from the phase III MARIANNE study. J. Clin. Oncol. 35, 141–148 (2017).
Untch, M. et al. LBA19 - Peripheral neuropathy (PN), thrombocytopenia (TCP) and central nervous system (CNS) recurrence: an update of the phase III KATHERINE trial of post-neoadjuvant trastuzumab emtansine (T-DM1) or trastuzumab (H) in patients (pts) with residual invasive HER2-positive breast cancer (BC). Ann. Oncol. 30, v854–v855 (2019).
Perez, E. A. et al. Sequential versus concurrent trastuzumab in adjuvant chemotherapy for breast cancer. J. Clin. Oncol. 29, 4491–4497 (2011).
Perez, E. A. et al. Four-year follow-up of trastuzumab plus adjuvant chemotherapy for operable human epidermal growth factor receptor 2-positive breast cancer: joint analysis of data from NCCTG N9831 and NSABP B-31. J. Clin. Oncol. 29, 3366–3373 (2011).
Joensuu, H. et al. Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N. Engl. J. Med. 354, 809–820 (2006).
Romond, E. H. et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N. Engl. J. Med. 353, 1673–1684 (2005).
Untch, M. et al. Lapatinib versus trastuzumab in combination with neoadjuvant anthracycline-taxane-based chemotherapy (GeparQuinto, GBG 44): a randomised phase 3 trial. Lancet Oncol. 13, 135–144 (2012).
Chen, S., Liang, Y., Feng, Z. & Wang, M. Efficacy and safety of HER2 inhibitors in combination with or without pertuzumab for HER2-positive breast cancer: a systematic review and meta-analysis. BMC Cancer 19, 973 (2019).
Zhu, C. et al. Safety and efficacy evaluation of pertuzumab in patients with solid tumors. Medicine 96, e6870 (2017).
Xin, Y. et al. Effects of lapatinib or trastuzumab, alone and in combination, in human epidermal growth factor receptor 2-positive breast cancer: a meta-analysis of randomized controlled trials. Cancer Med. 5, 3454–3463 (2016).
Acknowledgements
A.E. is supported by the Graduate Diploma in Health Research at the University of Toronto. S.D. is supported by an Early Researcher Award from the Province of Ontario. S.H., C.H., K.J.J. received no specific funding for this work.
Author information
Authors and Affiliations
Contributions
Project design: A.E. and S.D. Literature search and screening: A.E. and C.H. Data extraction: A.E. Statistical analysis: A.E. Manuscript draft and editing: A.E., S.H., K.J., and S.D. Project supervision: S.D.
Corresponding author
Ethics declarations
Competing interests
K.J.J. is a consultant and/or speaker for Apobiologix, Amgen, Esai, Genomic Health Inc., Novartis, Purdue Pharma, Pfizer, Roche. A.E., S.H., C.H., and S.D. declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Erickson, A.W., Habbous, S., Hoey, C. et al. Dual- versus single-agent HER2 inhibition and incidence of intracranial metastatic disease: a systematic review and meta-analysis. npj Breast Cancer 7, 17 (2021). https://doi.org/10.1038/s41523-021-00220-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41523-021-00220-0