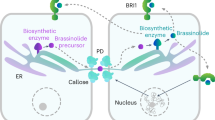
Abstract
Adjusting the microenvironment around the cell surface is critical to responding to external cues or endogenous signals and to maintaining cell activities. In plant cells, the plasma membrane is covered by the cell wall and scaffolded with cytoskeletal networks, which altogether compose the cell surface. It has long been known that these structures mutually interact, but the mechanisms that integrate the whole system are still obscure. Here we spotlight the brassinosteroid (BR) plant hormone receptor BRASSINOSTEROID INSENSITIVE1 (BRI1) since it represents an outstanding model for understanding cell surface signalling and regulation. We summarize how BRI1 activity and dynamics are controlled by plasma membrane components and their associated factors to fine-tune signalling. The downstream signals, in turn, manipulate cell surface structures by transcriptional and post-translational mechanisms. Moreover, the changes in these architectures impact BR signalling, resulting in a feedback loop formation. This Review discusses how BRI1 and BR signalling function as central hubs to integrate cell surface regulation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Congreve, M., de Graaf, C., Swain, N. A. & Tate, C. G. Impact of GPCR structures on drug discovery. Cell 181, 81–91 (2020).
Robinson, D. R., Wu, Y.-M. & Lin, S.-F. The protein tyrosine kinase family of the human genome. Oncogene 19, 5548–5557 (2000).
Shiu, S.-H. & Bleecker, A. B. Receptor-like kinases from Arabidopsis form a monophyletic gene family related to animal receptor kinases. Proc. Natl Acad. Sci. USA 98, 10763–10768 (2001).
Shiu, S.-H. et al. Comparative analysis of the receptor-like kinase family in Arabidopsis and rice. Plant Cell 16, 1220–1234 (2004).
Li, J. & Chory, J. A putative leucine-rich repeat receptor kinase involved in brassinosteroid signal transduction. Cell 90, 929–938 (1997).
Choe, S. et al. The Arabidopsis dwf7/ste1 mutant is defective in the Δ7 Sterol C-5 desaturation step leading to brassinosteroid biosynthesis. Plant Cell 11, 207–221 (1999).
Nakaya, M., Tsukaya, H., Murakami, N. & Kato, M. Brassinosteroids control the proliferation of leaf cells of Arabidopsis thaliana. Plant Cell Physiol. 43, 239–244 (2002).
Savaldi-Goldstein, S., Peto, C. & Chory, J. The epidermis both drives and restricts plant shoot growth. Nature 446, 199–202 (2007).
Ye, Q. et al. Brassinosteroids control male fertility by regulating the expression of key genes involved in Arabidopsis anther and pollen development. Proc. Natl Acad. Sci. USA 107, 6100–6105 (2010).
Vogler, F., Schmalzl, C., Englhart, M., Bircheneder, M. & Sprunck, S. Brassinosteroids promote Arabidopsis pollen germination and growth. Plant Reprod. 27, 153–167 (2014).
Krishna, P., Prasad, B. D. & Rahman, T. in Brassinosteroids: Methods and Protocols (eds Russinova, E. & Caño-Delgado, A. I.) 193–202 (Springer, 2017); https://doi.org/10.1007/978-1-4939-6813-8_16
Planas-Riverola, A. et al. Brassinosteroid signaling in plant development and adaptation to stress. Development 146, dev151894 (2019).
Hong, Z. et al. Loss-of-function of a rice brassinosteroid biosynthetic enzyme, C-6 oxidase, prevents the organized arrangement and polar elongation of cells in the leaves and stem. Plant J. 32, 495–508 (2002).
Mori, M. et al. Isolation and characterization of a rice dwarf mutant with a defect in brassinosteroid biosynthesis. Plant Physiol. 130, 1152–1161 (2002).
Nakamura, A. et al. The role of OsBRI1 and its homologous genes, OsBRL1 and OsBRL3, in rice. Plant Physiol. 140, 580–590 (2006).
Song, L. et al. Reducing brassinosteroid signalling enhances grain yield in semi-dwarf wheat. Nature 617, 118–124 (2023).
Oh, M.-H. et al. Tyrosine phosphorylation of the BRI1 receptor kinase emerges as a component of brassinosteroid signaling in Arabidopsis. Proc. Natl Acad. Sci. USA 106, 658–663 (2009).
Bojar, D. et al. Crystal structures of the phosphorylated BRI1 kinase domain and implications for brassinosteroid signal initiation. Plant J. 78, 31–43 (2014).
Vert, G., Nemhauser, J. L., Geldner, N., Hong, F. & Chory, J. Molecular mechanisms of steroid hormone signaling in plants. Annu. Rev. Cell Dev. Biol. 21, 177–201 (2005).
Jaillais, Y. & Vert, G. Brassinosteroid signaling and BRI1 dynamics went underground. Curr. Opin. Plant Biol. 33, 92–100 (2016).
Nolan, T. M., Vukašinović, N., Liu, D., Russinova, E. & Yin, Y. Brassinosteroids: multidimensional regulators of plant growth, development, and stress responses. Plant Cell 32, 295–318 (2020).
Li, J., Nam, K. H., Vafeados, D. & Chory, J. BIN2, a new brassinosteroid-insensitive locus in Arabidopsis. Plant Physiol. 127, 14–22 (2001).
He, J.-X., Gendron, J. M., Yang, Y., Li, J. & Wang, Z.-Y. The GSK3-like kinase BIN2 phosphorylates and destabilizes BZR1, a positive regulator of the brassinosteroid signaling pathway in Arabidopsis. Proc. Natl Acad. Sci. USA 99, 10185–10190 (2002).
Zhao, J. et al. Two putative BIN2 substrates are nuclear components of brassinosteroid signaling. Plant Physiol. 130, 1221–1229 (2002).
Vert, G. & Chory, J. Downstream nuclear events in brassinosteroid signalling. Nature 441, 96–100 (2006).
Gampala, S. S. et al. An essential role for 14-3-3 proteins in brassinosteroid signal transduction in Arabidopsis. Dev. Cell 13, 177–189 (2007).
Yin, Y. et al. BES1 accumulates in the nucleus in response to brassinosteroids to regulate gene expression and promote stem elongation. Cell 109, 181–191 (2002).
Wang, Z. Y. et al. Nuclear-localized BZR1 mediates brassinosteroid-induced growth and feedback suppression of brassinosteroid biosynthesis. Dev. Cell 2, 505–513 (2002).
Ryu, H. et al. Nucleocytoplasmic shuttling of BZR1 mediated by phosphorylation is essential in Arabidopsis brassinosteroid signaling. Plant Cell 19, 2749–2762 (2007).
Wang, R. et al. Nucleocytoplasmic trafficking and turnover mechanisms of BRASSINAZOLE RESISTANT1 in Arabidopsis thaliana. Proc. Natl Acad. Sci. USA 118, e2101838118 (2021).
Li, J. et al. BAK1, an Arabidopsis LRR receptor-like protein kinase, interacts with BRI1 and modulates brassinosteroid signaling. Cell 110, 213–222 (2002).
Nam, K. H. & Li, J. BRI1/BAK1, a receptor kinase pair mediating brassinosteroid signaling. Cell 110, 203–212 (2002).
Santiago, J., Henzler, C. & Hothorn, M. Molecular mechanism for plant steroid receptor activation by somatic embryogenesis co-receptor kinases. Science 341, 889–892 (2013).
Wang, X. et al. Sequential transphosphorylation of the BRI1/BAK1 receptor kinase complex impacts early events in brassinosteroid signaling. Dev. Cell 15, 220–235 (2008).
Tang, W. et al. Brassinosteroid-Signaling Kinases (BSKs) mediate signal transduction from the receptor kinase BRI1 in Arabidopsis. Science 321, 557–560 (2008).
Kim, T.-W., Guan, S., Burlingame, A. L. & Wang, Z.-Y. The CDG1 kinase mediates brassinosteroid signal transduction from BRI1 receptor kinase to BSU1 phosphatase and GSK3-like kinase BIN2. Mol. Cell 43, 561–571 (2011).
Mora-García, S. et al. Nuclear protein phosphatases with Kelch-repeat domains modulate the response to brassinosteroids in Arabidopsis. Genes Dev. 18, 448–460 (2004).
Kim, T.-W. et al. Brassinosteroid signal transduction from cell-surface receptor kinases to nuclear transcription factors. Nat. Cell Biol. 11, 1254–1260 (2009).
Tang, W. et al. PP2A activates brassinosteroid-responsive gene expression and plant growth by dephosphorylating BZR1. Nat. Cell Biol. 13, 124–131 (2011).
Müssig, C., Fischer, S. & Altmann, T. Brassinosteroid-regulated gene expression. Plant Physiol. 129, 1241–1251 (2002).
Sun, Y. et al. Integration of brassinosteroid signal transduction with the transcription network for plant growth regulation in Arabidopsis. Dev. Cell 19, 765–777 (2010).
Yu, X. et al. A brassinosteroid transcriptional network revealed by genome-wide identification of BESI target genes in Arabidopsis thaliana. Plant J. 65, 634–646 (2011).
Kadota, Y. et al. Direct regulation of the NADPH oxidase RBOHD by the PRR-associated kinase BIK1 during plant immunity. Mol. Cell 54, 43–55 (2014).
Li, L. et al. The FLS2-associated kinase BIK1 directly phosphorylates the NADPH oxidase RbohD to control plant immunity. Cell Host Microbe 15, 329–338 (2014).
Fujita, S. et al. SCHENGEN receptor module drives localized ROS production and lignification in plant roots. EMBO J. 39, e103894 (2020).
Lin, W. et al. TMK-based cell-surface auxin signalling activates cell-wall acidification. Nature 599, 278–282 (2021).
Li, L. et al. Cell surface and intracellular auxin signalling for H+ fluxes in root growth. Nature 599, 273–277 (2021).
Viotti, C. et al. Endocytic and secretory traffic in Arabidopsis merge in the trans-Golgi network/early endosome, an independent and highly dynamic organelle. Plant Cell 22, 1344–1357 (2010).
Sutkeviciute, I. & Vilardaga, J.-P. Structural insights into emergent signaling modes of G protein–coupled receptors. J. Biol. Chem. 295, 11626–11642 (2020).
Di Rubbo, S. et al. The clathrin adaptor complex AP-2 mediates endocytosis of BRASSINOSTEROID INSENSITIVE1 in Arabidopsis. Plant Cell 25, 2986–2997 (2013).
Martins, S. et al. Internalization and vacuolar targeting of the brassinosteroid hormone receptor BRI1 are regulated by ubiquitination. Nat. Commun. 6, 6151 (2015).
Liu, D. et al. Endocytosis of BRASSINOSTEROID INSENSITIVE1 is partly driven by a canonical Tyr-based motif. Plant Cell 32, 3598–3612 (2020).
Saeed, B. et al. K63-linked ubiquitin chains are a global signal for endocytosis and contribute to selective autophagy in plants. Curr. Biol. 33, 1337–1345.e5 (2023).
Geldner, N., Hyman, D. L., Wang, X., Schumacher, K. & Chory, J. Endosomal signaling of plant steroid receptor kinase BRI1. Genes Dev. 21, 1598–1602 (2007).
Neubus Claus, L. A. et al. BRASSINOSTEROID INSENSITIVE1 internalization can occur independent of ligand binding. Plant Physiol. https://doi.org/10.1093/plphys/kiad005 (2023).
Russinova, E. et al. Heterodimerization and endocytosis of Arabidopsis brassinosteroid receptors BRI1 and AtSERK3 (BAK1). Plant Cell 16, 3216–3229 (2004).
Robatzek, S., Chinchilla, D. & Boller, T. Ligand-induced endocytosis of the pattern recognition receptor FLS2 in Arabidopsis. Genes Dev. 20, 537–542 (2006).
Doblas, V. G. et al. Root diffusion barrier control by a vasculature-derived peptide binding to the SGN3 receptor. Science 355, 280–284 (2017).
Zhou, J. et al. Regulation of Arabidopsis brassinosteroid receptor BRI1 endocytosis and degradation by plant U-box PUB12/PUB13-mediated ubiquitination. Proc. Natl Acad. Sci. USA 115, E1906–E1915 (2018).
Zhang, L. et al. SYP22 and VAMP727 regulate BRI1 plasma membrane targeting to control plant growth in Arabidopsis. N. Phytol. 223, 1059–1065 (2019).
Gadeyne, A. et al. The TPLATE adaptor complex drives clathrin-mediated endocytosis in plants. Cell 156, 691–704 (2014).
Wang, L. et al. Spatiotemporal dynamics of the BRI1 receptor and its regulation by membrane microdomains in living Arabidopsis cells. Mol. Plant 8, 1334–1349 (2015).
Yoshinari, A. et al. Polar localization of the borate exporter BOR1 requires AP2-dependent endocytosis. Plant Physiol. 179, 1569–1580 (2019).
Leitner, J. et al. Lysine63-linked ubiquitylation of PIN2 auxin carrier protein governs hormonally controlled adaptation of Arabidopsis root growth. Proc. Natl Acad. Sci. USA 109, 8322–8327 (2012).
Johnson, A. & Vert, G. Unraveling K63 polyubiquitination networks by sensor-based proteomics. Plant Physiol. 171, 1808–1820 (2016).
Dubeaux, G., Neveu, J., Zelazny, E. & Vert, G. Metal sensing by the IRT1 transporter-receptor orchestrates its own degradation and plant metal nutrition. Mol. Cell 69, 953–964.e5 (2018).
Luo, Y. et al. Deubiquitinating enzymes UBP12 and UBP13 stabilize the brassinosteroid receptor BRI1. EMBO Rep. 23, e53354 (2022).
Lu, D. et al. Direct ubiquitination of pattern recognition receptor FLS2 attenuates plant innate immunity. Science 332, 1439–1442 (2011).
Martins, S. et al. Brassinosteroid signaling-dependent root responses to prolonged elevated ambient temperature. Nat. Commun. 8, 309 (2017).
Naranjo-Arcos, M. et al. SUMO/deSUMOylation of the BRI1 brassinosteroid receptor modulates plant growth responses to temperature. Proc. Natl Acad. Sci. USA 120, e2217255120 (2023).
Orosa, B. et al. SUMO conjugation to the pattern recognition receptor FLS2 triggers intracellular signalling in plant innate immunity. Nat. Commun. 9, 5185 (2018).
Lin, W. et al. Inverse modulation of plant immune and brassinosteroid signaling pathways by the receptor-like cytoplasmic kinase BIK1. Proc. Natl Acad. Sci. USA 110, 12114–12119 (2013).
Heinemann, B., Künzler, P., Eubel, H., Braun, H.-P. & Hildebrandt, T. M. Estimating the number of protein molecules in a plant cell: protein and amino acid homeostasis during drought. Plant Physiol. 185, 385–404 (2021).
Platre, M. P. et al. Developmental control of plant Rho GTPase nano-organization by the lipid phosphatidylserine. Science 364, 57–62 (2019).
Smokvarska, M. et al. A plasma membrane nanodomain ensures signal specificity during osmotic signaling in plants. Curr. Biol. 30, 4654–4664.e4 (2020).
Gronnier, J. et al. Regulation of immune receptor kinase plasma membrane nanoscale organization by a plant peptide hormone and its receptors. eLife 11, e74162 (2022).
Chinchilla, D. et al. A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defence. Nature 448, 497–500 (2007).
Heese, A. et al. The receptor-like kinase SERK3/BAK1 is a central regulator of innate immunity in plants. Proc. Natl Acad. Sci. USA 104, 12217–12222 (2007).
Schulze, B. et al. Rapid heteromerization and phosphorylation of ligand-activated plant transmembrane receptors and their associated kinase BAK1. J. Biol. Chem. 285, 9444–9451 (2010).
Belkhadir, Y., Yang, L., Hetzel, J., Dangl, J. L. & Chory, J. The growth–defense pivot: crisis management in plants mediated by LRR-RK surface receptors. Trends Biochem. Sci. 39, 447–456 (2014).
Ortiz-Morea, F. A., He, P., Shan, L. & Russinova, E. It takes two to tango—molecular links between plant immunity and brassinosteroid signalling. J. Cell Sci. 133, jcs246728 (2020).
Bücherl, C. A. et al. Plant immune and growth receptors share common signalling components but localise to distinct plasma membrane nanodomains. eLife 6, e25114 (2017).
Su, B. et al. Dynamic spatial reorganization of BSK1 complexes in the plasma membrane underpins signal-specific activation for growth and immunity. Mol. Plant 14, 588–603 (2021).
Good, M. C., Zalatan, J. G. & Lim, W. A. Scaffold proteins: hubs for controlling the flow of cellular information. Science 332, 680–686 (2011).
Amorim-Silva, V. et al. TTL proteins scaffold brassinosteroid signaling components at the plasma membrane to optimize signal transduction in Arabidopsis. Plant Cell 31, 1807–1828 (2019).
Ren, H. et al. Brassinosteroid-signaling kinase 3, a plasma membrane-associated scaffold protein involved in early brassinosteroid signaling. PLoS Genet. 15, e1007904 (2019).
Lakhssassi, N. et al. The Arabidopsis tetratricopeptide thioredoxin-like gene family is required for osmotic stress tolerance and male sporogenesis. Plant Physiol. 158, 1252–1266 (2012).
Kesten, C. et al. Peripheral membrane proteins modulate stress tolerance by safeguarding cellulose synthases. Sci. Adv. 8, eabq6971 (2022).
Wang, X. et al. Autoregulation and homodimerization are involved in the activation of the plant steroid receptor BRI1. Dev. Cell 8, 855–865 (2005).
Hothorn, M. et al. Structural basis of steroid hormone perception by the receptor kinase BRI1. Nature 474, 467–471 (2011).
Hink, M. A., Shah, K., Russinova, E., de Vries, S. C. & Visser, A. J. W. G. Fluorescence fluctuation analysis of Arabidopsis thaliana somatic embryogenesis receptor-like kinase and brassinosteroid insensitive 1 receptor oligomerization. Biophys. J. 94, 1052–1062 (2008).
Wang, X. et al. Identification and functional analysis of in vivo phosphorylation sites of the Arabidopsis brassinosteroid-insensitive1 receptor kinase. Plant Cell 17, 1685–1703 (2005).
Oh, M.-H., Wang, X., Clouse, S. D. & Huber, S. C. Deactivation of the Arabidopsis brassinosteroid insensitive 1 (BRI1) receptor kinase by autophosphorylation within the glycine-rich loop. Proc. Natl Acad. Sci. USA 109, 327–332 (2012).
Liu, Y., Huang, X., Li, M., He, P. & Zhang, Y. Loss-of-function of Arabidopsis receptor-like kinase BIR1 activates cell death and defense responses mediated by BAK1 and SOBIR1. N. Phytol. 212, 637–645 (2016).
Ma, C. et al. Structural basis for BIR1-mediated negative regulation of plant immunity. Cell Res. 27, 1521–1524 (2017).
Halter, T. et al. The leucine-rich repeat receptor kinase BIR2 is a negative regulator of BAK1 in plant immunity. Curr. Biol. 24, 134–143 (2014).
Hohmann, U., Nicolet, J., Moretti, A., Hothorn, L. A. & Hothorn, M. The SERK3 elongated allele defines a role for BIR ectodomains in brassinosteroid signalling. Nat. Plants 4, 345–351 (2018).
Imkampe, J. et al. The Arabidopsis leucine-rich repeat receptor kinase BIR3 negatively regulates BAK1 receptor complex formation and stabilizes BAK1. Plant Cell 29, 2285–2303 (2017).
Wang, X. & Chory, J. Brassinosteroids regulate dissociation of BKI1, a negative regulator of BRI1 signaling, from the plasma membrane. Science 313, 1118–1122 (2006).
Jaillais, Y. et al. Tyrosine phosphorylation controls brassinosteroid receptor activation by triggering membrane release of its kinase inhibitor. Genes Dev. 25, 232–237 (2011).
Simon, M. L. A. et al. A PtdIns(4)P-driven electrostatic field controls cell membrane identity and signalling in plants. Nat. Plants 2, 16089 (2016).
Kang, Y. H. & Hardtke, C. S. Arabidopsis MAKR5 is a positive effector of BAM3-dependent CLE45 signaling. EMBO Rep. 17, 1145–1154 (2016).
Marquès-Bueno, M. M. et al. Auxin-regulated reversible inhibition of TMK1 signaling by MAKR2 modulates the dynamics of root gravitropism. Curr. Biol. 31, 228–237.e10 (2021).
Wang, D. et al. BKI1 regulates plant architecture through coordinated inhibition of the brassinosteroid and ERECTA signaling pathways in Arabidopsis. Mol. Plant 10, 297–308 (2017).
Wu, G. et al. Methylation of a phosphatase specifies dephosphorylation and degradation of activated brassinosteroid receptors. Sci. Signal. 4, ra29 (2011).
Wang, R. et al. The brassinosteroid-activated BRI1 receptor kinase is switched off by dephosphorylation mediated by cytoplasm-localized PP2A B′ subunits. Mol. Plant 9, 148–157 (2016).
Liu, X. et al. Salicylic acid attenuates brassinosteroid signaling via protein de-S-acylation. EMBO J. 42, e112998 (2023).
Wang, H. et al. Abscisic acid signaling inhibits brassinosteroid signaling through dampening the dephosphorylation of BIN2 by ABI1 and ABI2. Mol. Plant 11, 315–325 (2018).
Kost, B. & Chua, N.-H. The plant cytoskeleton: vacuoles and cell walls make the difference. Cell 108, 9–12 (2002).
Hamant, O. et al. Developmental patterning by mechanical signals in Arabidopsis. Science 322, 1650–1655 (2008).
Fujita, S. et al. An atypical tubulin kinase mediates stress-induced microtubule depolymerization in Arabidopsis. Curr. Biol. 23, 1969–1978 (2013).
Oda, Y. & Fukuda, H. Initiation of cell wall pattern by a rho- and microtubule-driven symmetry breaking. Science 337, 1333–1336 (2012).
Lindeboom, J. J. et al. A mechanism for reorientation of cortical microtubule arrays driven by microtubule severing. Science 342, 1245533 (2013).
Qian, D. et al. Arabidopsis ADF5 promotes stomatal closure by regulating actin cytoskeleton remodeling in response to ABA and drought stress. J. Exp. Bot. 70, 435–446 (2019).
Takatani, S. et al. Microtubule response to tensile stress is curbed by NEK6 to buffer growth variation in the Arabidopsis hypocotyl. Curr. Biol. 30, 1491–1503.e2 (2020).
Catterou, M. et al. Brassinosteroids, microtubules and cell elongation in Arabidopsis thaliana. II. Effects of brassinosteroids on microtubules and cell elongation in the bul1 mutant. Planta 212, 673–683 (2001).
Lanza, M. et al. Role of actin cytoskeleton in brassinosteroid signaling and in its integration with the auxin response in plants. Dev. Cell 22, 1275–1285 (2012).
de Bang, L. et al. Brassinosteroids inhibit autotropic root straightening by modifying filamentous-actin organization and dynamics. Front. Plant Sci. 11, 5 (2020).
Blume, Ya. B., Krasylenko, Yu. A. & Yemets, A. I. Effects of phytohormones on the cytoskeleton of the plant cell. Russ. J. Plant Physiol. 59, 515–529 (2012).
Wang, X. et al. Arabidopsis microtubule destabilizing protein40 is involved in brassinosteroid regulation of hypocotyl elongation. Plant Cell 24, 4012–4025 (2012).
Wang, J. et al. Brassinosteroid signals cooperate with katanin-mediated microtubule severing to control stamen filament elongation. EMBO J. 42, e111883 (2022).
Ambrose, C., Allard, J. F., Cytrynbaum, E. N. & Wasteneys, G. O. A CLASP-modulated cell edge barrier mechanism drives cell-wide cortical microtubule organization in Arabidopsis. Nat. Commun. 2, 430 (2011).
Ruan, Y. et al. The microtubule-associated protein CLASP sustains cell proliferation through a brassinosteroid signaling negative feedback loop. Curr. Biol. 28, 2718–2729.e5 (2018).
Ambrose, C. et al. CLASP interacts with sorting nexin 1 to link microtubules and auxin transport via PIN2 recycling in Arabidopsis thaliana. Dev. Cell 24, 649–659 (2013).
Jaillais, Y., Fobis-Loisy, I., Miège, C. & Gaude, T. Evidence for a sorting endosome in Arabidopsis root cells. Plant J. 53, 237–247 (2008).
De Rybel, B. et al. Chemical inhibition of a subset of Arabidopsis thaliana GSK3-like kinases activates brassinosteroid signaling. Chem. Biol. 16, 594–604 (2009).
Min, Y. K. et al. New lead compounds for brassinosteroid biosynthesis inhibitors. Bioorg. Med. Chem. Lett. 9, 425–430 (1999).
Liu, X. et al. Brassinosteroids regulate pavement cell growth by mediating BIN2-induced microtubule stabilization. J. Exp. Bot. 69, 1037–1049 (2018).
Zhang, C. et al. ROPGAP-dependent interaction between brassinosteroid and ROP2–GTPase signaling controls pavement cell shape in Arabidopsis. Curr. Biol. 32, 518–531.e6 (2022).
Lauster, T. et al. Arabidopsis pavement cell shape formation involves spatially confined ROPGAP regulators. Curr. Biol. 32, 532–544.e7 (2022).
Feiguelman, G., Fu, Y. & Yalovsky, S. ROP GTPases structure–function and signaling pathways. Plant Physiol. 176, 57–79 (2018).
Fu, Y., Gu, Y., Zheng, Z., Wasteneys, G. & Yang, Z. Arabidopsis interdigitating cell growth requires two antagonistic pathways with opposing action on cell morphogenesis. Cell 120, 687–700 (2005).
Lin, D. et al. Rho GTPase signaling activates microtubule severing to promote microtubule ordering in Arabidopsis. Curr. Biol. 23, 290–297 (2013).
Deng, Z. et al. A proteomic study of brassinosteroid response in Arabidopsis. Mol. Cell. Proteom. 6, 2058–2071 (2007).
Kim, T.-W. et al. Mapping the signaling network of BIN2 kinase using TurboID-mediated biotin labeling and phosphoproteomics. Plant Cell 35, 975–993 (2023).
Sánchez-Rodríguez, C. et al. Brassinosteroid insensitive2 negatively regulates cellulose synthesis in Arabidopsis by phosphorylating cellulose synthase 1. Proc. Natl Acad. Sci. USA 114, 3533–3538 (2017).
Somssich, M. et al. Brassinosteroids influence Arabidopsis hypocotyl graviresponses through changes in mannans and cellulose. Plant Cell Physiol. 62, 678–692 (2021).
Wang, W., Sun, Y., Li, G. & Zhang, S. Brassinosteroids promote parenchyma cell and secondary xylem development in sugar beet (Beta vulgaris L.) root. Plant Direct 5, e340 (2021).
Li, M. et al. Brassinosteroid signaling restricts root lignification by antagonizing SHORT-ROOT function in Arabidopsis. Plant Physiol. 190, 1182–1198 (2022).
Zhuang, Y. et al. MYB42 inhibits hypocotyl cell elongation by coordinating brassinosteroid homeostasis and signalling in Arabidopsis thaliana. Ann. Bot. 129, 403–413 (2021).
Vatén, A. et al. Callose biosynthesis regulates symplastic trafficking during root development. Dev. Cell 21, 1144–1155 (2011).
Sager, R. E. & Lee, J.-Y. Plasmodesmata at a glance. J. Cell Sci. 131, jcs209346 (2018).
Wang, Y. et al. Plasmodesmata mediate cell-to-cell transport of brassinosteroid hormones. Nat. Chem. Biol. https://doi.org/10.1038/s41589-023-01346-x (2023).
Clouse, S. D. Brassinosteroids. Arabidopsis Book 1, e0009 (2002).
Vukašinović, N. et al. Local brassinosteroid biosynthesis enables optimal root growth. Nat. Plants 7, 619–632 (2021).
Takahashi, K., Hirata, S., Kido, N. & Katou, K. Wall-yielding properties of cell walls from elongating cucumber hypocotyls in relation to the action of expansin. Plant Cell Physiol. 47, 1520–1529 (2006).
Friml, J. et al. ABP1–TMK auxin perception for global phosphorylation and auxin canalization. Nature 609, 575–581 (2022).
Spartz, A. K. et al. SAUR inhibition of PP2C-D phosphatases activates plasma membrane H+-ATPases to promote cell expansion in Arabidopsis. Plant Cell 26, 2129–2142 (2014).
Caesar, K. et al. A fast brassinolide-regulated response pathway in the plasma membrane of Arabidopsis thaliana. Plant J. 66, 528–540 (2011).
Minami, A., Takahashi, K., Inoue, S., Tada, Y. & Kinoshita, T. Brassinosteroid induces phosphorylation of the plasma membrane H+-ATPase during hypocotyl elongation in Arabidopsis thaliana. Plant Cell Physiol. 60, 935–944 (2019).
Li, M. et al. SAUR15 interaction with BRI1 activates plasma membrane H+-ATPase to promote organ development of Arabidopsis. Plant Physiol. 189, 2454–2466 (2022).
Großeholz, R. et al. Computational modeling and quantitative physiology reveal central parameters for brassinosteroid-regulated early cell physiological processes linked to elongation growth of the Arabidopsis root. eLife 11, e73031 (2022).
Liu, L. et al. Extracellular pH sensing by plant cell-surface peptide-receptor complexes. Cell 185, 3341–3355.e13 (2022).
Diaz-Ardila, H. N., Gujas, B., Wang, Q., Moret, B. & Hardtke, C. S. pH-dependent CLE peptide perception permits phloem differentiation in Arabidopsis roots. Curr. Biol. 33, 597–605.e3 (2023).
Doblas, V. G., Gonneau, M. & Höfte, H. Cell wall integrity signaling in plants: malectin-domain kinases and lessons from other kingdoms. Cell Surf. 3, 1–11 (2018).
Mishra, R., Minc, N. & Peter, M. Cells under pressure: how yeast cells respond to mechanical forces. Trends Microbiol. 30, 495–510 (2022).
Engelsdorf, T. et al. The plant cell wall integrity maintenance and immune signaling systems cooperate to control stress responses in Arabidopsis thaliana. Sci. Signal. 11, eaao3070 (2018).
Wolf, S. Cell wall signaling in plant development and defense. Annu. Rev. Plant Biol. 73, 323–353 (2022).
Haswell, E. S., Peyronnet, R., Barbier-Brygoo, H., Meyerowitz, E. M. & Frachisse, J.-M. Two MscS homologs provide mechanosensitive channel activities in the Arabidopsis root. Curr. Biol. 18, 730–734 (2008).
Mecchia, M. A. et al. RALF4/19 peptides interact with LRX proteins to control pollen tube growth in Arabidopsis. Science 358, 1600–1603 (2017).
Wolf, S. et al. A receptor-like protein mediates the response to pectin modification by activating brassinosteroid signaling. Proc. Natl Acad. Sci. USA 111, 15261–15266 (2014).
Ge, Z. et al. LLG2/3 are co-receptors in BUPS/ANX–RALF signaling to regulate Arabidopsis pollen tube integrity. Curr. Biol. 29, 3256–3265.e5 (2019).
Hématy, K. et al. A receptor-like kinase mediates the response of Arabidopsis cells to the inhibition of cellulose synthesis. Curr. Biol. 17, 922–931 (2007).
Miyazaki, S. et al. ANXUR1 and 2, sister genes to FERONIA/SIRENE, are male factors for coordinated fertilization. Curr. Biol. 19, 1327–1331 (2009).
Tang, W. et al. Mechano-transduction via the pectin–FERONIA complex activates ROP6 GTPase signaling in Arabidopsis pavement cell morphogenesis. Curr. Biol. 32, 508–517.e3 (2022).
Ge, Z. et al. Arabidopsis pollen tube integrity and sperm release are regulated by RALF-mediated signaling. Science 358, 1596–1600 (2017).
Haas, K. T., Wightman, R., Meyerowitz, E. M. & Peaucelle, A. Pectin homogalacturonan nanofilament expansion drives morphogenesis in plant epidermal cells. Science 367, 1003–1007 (2020).
Wolf, S., Mravec, J., Greiner, S., Mouille, G. & Höfte, H. Plant cell wall homeostasis is mediated by brassinosteroid feedback signaling. Curr. Biol. 22, 1732–1737 (2012).
Holzwart, E. et al. A mutant allele uncouples the brassinosteroid-dependent and independent functions of brassinosteroid insensitive 11. Plant Physiol. 182, 669–678 (2020).
Garnelo Gómez, B., Holzwart, E., Shi, C., Lozano-Durán, R. & Wolf, S. Phosphorylation-dependent routing of RLP44 towards brassinosteroid or phytosulfokine signalling. J. Cell Sci. 134, jcs259134 (2021).
Holzwart, E. et al. BRI1 controls vascular cell fate in the Arabidopsis root through RLP44 and phytosulfokine signaling. Proc. Natl Acad. Sci. USA 115, 11838–11843 (2018).
Yue, Z.-L. et al. The receptor kinase OsWAK11 monitors cell wall pectin changes to fine-tune brassinosteroid signaling and regulate cell elongation in rice. Curr. Biol. 32, 2454–2466.e7 (2022).
Huerta, A. I. et al. The WAK-like protein RFO1 acts as a sensor of the pectin methylation status in Arabidopsis cell walls to modulate root growth and defense. Mol. Plant 16, 865–881 (2023).
Ubeda-Tomás, S. et al. Root growth in Arabidopsis requires gibberellin/DELLA signalling in the endodermis. Nat. Cell Biol. 10, 625–628 (2008).
Uchida, N. et al. Regulation of inflorescence architecture by intertissue layer ligand–receptor communication between endodermis and phloem. Proc. Natl Acad. Sci. USA 109, 6337–6342 (2012).
Fridman, Y. et al. Root growth is modulated by differential hormonal sensitivity in neighboring cells. Genes Dev. 28, 912–920 (2014).
Fridman, Y. et al. The root meristem is shaped by brassinosteroid control of cell geometry. Nat. Plants 7, 1475–1484 (2021).
Kelly-Bellow, R. et al. Brassinosteroid coordinates cell layer interactions in plants via cell wall and tissue mechanics. Science 380, 1275–1281 (2023).
Azpiroz, R., Wu, Y., LoCascio, J. C. & Feldmann, K. A. An Arabidopsis brassinosteroid-dependent mutant is blocked in cell elongation. Plant Cell 10, 219–230 (1998).
Choe, S. et al. The DWF4 gene of Arabidopsis encodes a cytochrome P450 that mediates multiple 22alpha-hydroxylation steps in brassinosteroid biosynthesis. Plant Cell 10, 231–243 (1998).
Grove, M. D. et al. Brassinolide, a plant growth-promoting steroid isolated from Brassica napus pollen. Nature 281, 216–217 (1979).
Acknowledgements
We apologize to authors whose work is related to BR-driven cell surface regulation but could not be cited because of either our oversight or space limitation. We thank S. Moussu for the critical reading of the manuscript. This work was supported by a PhD fellowship from the University Toulouse 3-Paul Sabatier to C.D., research grants from Agence Nationale de la Recherche (ANR-17-CE20-0026-01 to G.V. and ANR-22-CE13-0021-01 to S.F.) and the French Laboratory of Excellence (project ‘TULIP’ grant nos ANR-10-LABX-41 and ANR-11-IDEX-0002-02 to G.V.).
Author information
Authors and Affiliations
Contributions
C.D., G.V. and S.F. conceptualized the structure of the paper. C.D. wrote the original draft of the paper. C.D., G.V. and S.F. reviewed and edited the manuscript. C.D. and S.F. produced the figures. S.F. and G.V. supervised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Plants thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Delesalle, C., Vert, G. & Fujita, S. The cell surface is the place to be for brassinosteroid perception and responses. Nat. Plants 10, 206–218 (2024). https://doi.org/10.1038/s41477-024-01621-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-024-01621-2