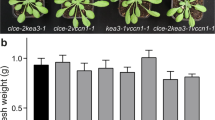
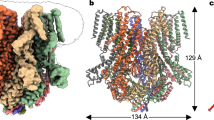
Abstract
Stomata of plant leaves open to enable CO2 entry for photosynthesis and close to reduce water loss via transpiration. Compared with photosynthesis, stomata respond slowly to fluctuating light, reducing assimilation and water use efficiency. Efficiency gains are possible without a cost to photosynthesis if stomatal kinetics can be accelerated. Here we show that clustering of the GORK channel, which mediates K+ efflux for stomatal closure in the model plant Arabidopsis, arises from binding between the channel voltage sensors, creating an extended ‘sensory antenna’ for channel gating. Mutants altered in clustering affect channel gating to facilitate K+ flux, accelerate stomatal movements and reduce water use without a loss in biomass. Our findings identify the mechanism coupling channel clustering with gating, and they demonstrate the potential for engineering of ion channels native to the guard cell to enhance stomatal kinetics and improve water use efficiency without a cost in carbon fixation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Data generated and analysed during this study are included in the article, its supplementary information files, and are also available on reasonable request to the corresponding author. Source data are provided with this paper.
Code availability
The OnGuard3 platform and the model parameter sets are freely available to academic users and may be downloaded from www.psrg.org.uk.
References
Hetherington, A. M. & Woodward, F. I. The role of stomata in sensing and driving environmental change. Nature 424, 901–908 (2003).
Lawson, T. & Blatt, M. R. Stomatal size, speed, and responsiveness impact on photosynthesis and water use efficiency. Plant Physiol. 164, 1556–1570 (2014).
Beljaars, A. C. M., Viterbo, P., Miller, M. J. & Betts, A. K. The anomalous rainfall over the United States during July 1993: sensitivity to land surface parameterization and soil moisture. Mon. Weather Rev. 124, 362–383 (1996).
Berry, J. A., Beerling, D. J. & Franks, P. J. Stomata: key players in the Earth system, past and present. Curr. Opin. Plant Biol. 13, 233–240 (2010).
Water for a Sustainable World—UN World Water Development Report. Vol. 1 (UNESCO, 2015).
Jezek, M. & Blatt, M. R. The membrane transport system of the guard cell and its integration for stomatal dynamics. Plant Physiol. 174, 487–519 (2017).
Assmann, S. M. & Jegla, T. Guard cell sensory systems: recent insights on stomatal responses to light, abscisic acid, and CO2. Curr. Opin. Plant Biol. 33, 157–167 (2016).
Nakamura, R. L. et al. Expression of an Arabidopsis potassium channel gene in guard cells. Plant Physiol. 109, 371–374 (1995).
Pilot, G., Gaymard, F., Mouline, K., Cherel, I. & Sentenac, H. Regulated expression of Arabidopsis Shaker K+ channel genes involved in K+ uptake and distribution in the plant. Plant Mol. Biol. 51, 773–787 (2003).
Lebaudy, A. et al. Plant adaptation to fluctuating environment and biomass production are strongly dependent on guard cell potassium channels. Proc. Natl Acad. Sci. USA 105, 5271–5276 (2008).
Pilot, G. et al. Guard cell inward K+ channel activity in Arabidopsis involves expression of the twin channel subunits KAT1 and KAT2. J. Biol. Chem. 276, 3215–3221 (2001).
Clint, G. M. & Blatt, M. R. Mechanisms of fusicoccin action: evidence for concerted modulations of secondary K+ transport in a higher-plant cell. Planta 178, 495–508 (1989).
Blatt, M. R. & Clint, G. M. Mechanisms of fusicoccin action kinetic modification and inactivation of potassium channels in guard cells. Planta 178, 509–523 (1989).
Maathuis, F. J. M. & Sanders, D. Mechanism of high-affinity potassium uptake in roots of Arabidopsis thaliana. Proc. Natl Acad. Sci. USA 91, 9272–9276 (1994).
Very, A.-A. et al. Molecular biology of K+ transport across the plant cell membrane: what do we learn from comparison between plant species? J. Plant Physiol. 171, 748–769 (2014).
Hosy, E. et al. The Arabidopsis outward K+ channel GORK is involved in regulation of stomatal movements and plant transpiration. Proc. Natl Acad. Sci. USA 100, 5549–5554 (2003).
Ache, P. et al. GORK, a delayed outward rectifier expressed in guard cells of Arabidopsis thaliana, is a K+-selective, K+-sensing ion channel. FEBS Lett. 486, 93–98 (2000).
Blatt, M. R. Potassium-dependent bipolar gating of potassium channels in guard cells. J. Membr. Biol. 102, 235–246 (1988).
Blatt, M. R. & Gradmann, D. K+-sensitive gating of the K+ outward rectifier in Vicia guard cells. J. Membr. Biol. 158, 241–256 (1997).
Eisenach, C., Papanatsiou, M., Hillert, E. K. & Blatt, M. R. Clustering of the K+ channel GORK of Arabidopsis parallels its gating by extracellular K+. Plant J. 78, 203–214 (2014).
Gaymard, F. et al. Identification and disruption of a plant shaker-like outward channel involved in K+ release into the xylem sap. Cell 94, 647–655 (1998).
Johansson, I. et al. External K+ modulates the activity of the Arabidopsis potassium channel SKOR via an unusual mechanism. Plant J. 46, 269–281 (2006).
Riedelsberger, J. et al. Distributed structures underlie gating differences between the Kin channel KAT1 and the Kout channel SKOR. Mol. Plant 3, 236–245 (2010).
Gajdanowicz, P. et al. Distributed structures determine K+ and voltage dependent gating of the Kin channel KAT1 and the Kout channel SKOR. N. Phytol. 182, 380–391 (2009).
Jegla, T., Busey, G. & Assmann, S. M. Evolution and structural characteristics of plant voltage-gated K+ channels. Plant Cell 30, 2898–2909 (2018).
Pozdnyakov, I., Safonov, P. & Skarlato, S. Diversity of voltage-gated potassium channels and cyclic nucleotide-binding domain-containing channels in eukaryotes. Sci. Rep. 10, 13 (2020).
Dreyer, I. & Blatt, M. R. What makes a gate? The ins and outs of Kv-like K+ channels in plants. Trends Plant Sci. 14, 383–390 (2009).
Very, A. A. & Sentenac, H. Cation channels in the Arabidopsis plasma membrane. Trends Plant Sci. 7, 168–175 (2002).
Yellen, G. The voltage-gated potassium channels and their relatives. Nature 419, 35–42 (2002).
Palovcak, E., Delemotte, L., Klein, M. L. & Carnevale, V. Evolutionary imprint of activation: the design principles of VSDs. J. Gen. Physiol. 143, 145–156 (2014).
Lefoulon, C. The bare necessities of plant K+ channel regulation. Plant Physiol. 187, 2092–2109 (2021).
Lefoulon, C. et al. Voltage-sensor transitions of the inward-rectifying K+ channel KAT1 indicate a closed-to-open latching mechanism that is biased by hydration around the S4 α-helix. Plant Physiol. 166, 960–975 (2014).
Grefen, C. et al. A vesicle-trafficking protein commandeers Kv channel voltage sensors for voltage-dependent secretion. Nat. Plants 1, 15108–15119 (2015).
Honsbein, A. et al. A tripartite SNARE-K+ channel complex mediates in channel-dependent K+ nutrition in Arabidopsis. Plant Cell 21, 2859–2877 (2009).
Grefen, C., Obrdlik, P. & Harter, K. The determination of protein–protein interactions byt the mating-based split-ubiquitin system (mbSUS). Methods Mol. Biol. 479, 1–17 (2009).
Grefen, C. & Blatt, M. R. A 2in1 cloning system enables ratiometric bimolecular fluorescence complementation (rBiFC). Biotechniques 53, 311–314 (2012).
Fricker, M. D., Runions, J. & Moore, I. Quantitative fluorescence microscopy: from art to science. Ann. Rev. Plant Biol. 57, 79–107 (2006).
Gould, G. W. Membrane Protein Expression Systems. Vol. 1 (Portland Press, 1994).
Lefoulon, C., Waghmare, S., Karnik, R. & Blatt, M. R. Gating control and K+ uptake by the KAT1 K+ channel leaveraged through membrane anchoring of the trafficking protein SYP121. Plant Cell Environ. 41, 2668–2677 (2018).
Grefen, C. et al. A ubiquitin-10 promoter-based vector set for fluorescent protein tagging facilitates temporal stability and native protein distribution in transient and stable expression studies. Plant J. 64, 355–365 (2010).
Yang, Y. Z., Costa, A., Leonhardt, N., Siegel, R. S. & Schroeder, J. I. Isolation of a strong Arabidopsis guard cell promoter and its potential as a research tool. Plant Methods 4, 4–6 (2008).
Wang, Y. et al. Unexpected connections between humidity and ion transport discovered using a model to bridge guard cell-to-leaf scales. Plant Cell 29, 2921–2139 (2017).
Wang, Y. et al. Systems dynamic modelling of a guard cell Cl− channel mutant uncovers an emergent homeostatic network regulating stomatal transpiration. Plant Physiol. 160, 1956–1972 (2012).
Eisenach, C., Chen, Z. H., Grefen, C. & Blatt, M. R. The trafficking protein SYP121 of Arabidopsis connects programmed stomatal closure and K+ channel acivity with vegetative growth. Plant J. 69, 241–251 (2012).
Blatt, M. R. K+ channels of stomatal guard cells: characteristics of the inward rectifier and its control by pH. J. Gen. Physiol. 99, 615–644 (1992).
Jezek, M. et al. Guard cell endomembrane Ca2+-ATPases underpin a ‘carbon memory’ of photosynthetic assimilation that impacts on water use efficiency. Nat. Plants 7, 1301–1307 (2021).
Becker, D. et al. Regulation of the ABA-sensitive Arabidopsis potassium channel gene GORK in response to water stress. FEBS Lett. 554, 119–126 (2003).
Papanatsiou, M. et al. Optogenetic manipulation of stomatal kinetics improves carbon assimilation and water use efficiency. Science 363, 1456–1459 (2019).
Sharkey, T. D., Bernacchi, C. J., Farquhar, G. D. & Singsaas, E. L. Fitting photosynthetic carbon dioxide response curves for C-3 leaves. Plant Cell Environ. 30, 1035–1040 (2007).
Tester, M. Plant ion channels: whole-cell and single-channel studies. N. Phytol. 114, 305–340 (1990).
Lai, H. C. & Jan, L. Y. The distribution and targeting of neuronal voltage-gated ion channels. Nat. Rev. Neurosci. 7, 548–562 (2006).
Deutsch, E. et al. Kv2.1 cell surface clusters are insertion platforms for ion channel delivery to the plasma membrane. Mol. Biol. Cell 23, 2917–2929 (2012).
Tamkun, M. M., O’Connell, K. M. S. & Rolig, A. S. A cytoskeletal-based perimeter fence selectively corrals a sub-population of cell surface Kv2.1 channels. J. Cell Sci. 120, 2413–2423 (2007).
Sutter, J. U., Campanoni, P., Tyrrell, M. & Blatt, M. R. Selective mobility and sensitivity to SNAREs is exhibited by the Arabidopsis KAT1 K+ channel at the plasma membrane. Plant Cell 18, 935–954 (2006).
Sutter, J. U. et al. Abscisic acid triggers the endocytosis of the Arabidopsis KAT1 K+ channel and its recycling to the plasma membrane. Curr. Biol. 17, 1396–1402 (2007).
Karnik, R. et al. Commandeering channel voltage sensors for secretion, cell turgor, and volume control. Trends Plant Sci. 11, 81–95 (2017).
Thiel, G., MacRobbie, E. A. C. & Blatt, M. R. Membrane transport in stomatal guard cells: the importance of voltage control. J. Membr. Biol. 126, 1–18 (1992).
Blatt, M. R. Potassium channel currents in intact stomatal guard cells: rapid enhancement by abscisic acid. Planta 180, 445–455 (1990).
Horaruang, W. The Mechanism of GORK K+ Channel Gating and Clustering. PhD thesis, Univ. Glasgow (2019).
Dickinson, M. S., Pourmal, S., Gupta, M., Bi, M. & Stroud, R. M. Symmetry reduction in a hyperpolarization-activated homotetrameric ion channel. Biochemistry in press, https://doi.org/10.1021/acs.biochem.1c00654 (2022).
Lee, C.-H. & MacKinnon, R. Structures of the human HCN1 hyperpolarization-activated channel. Cell 168, 111 (2017).
Porro, A. et al. The HCN domain couples voltage gating and cAMP response in hyperpolarization-activated cyclic nucleotide-gated channels. eLife 8, e49672 (2019).
Doheny-Adams, T., Hunt, L., Franks, P. J., Beerling, D. J. & Gray, J. E. Genetic manipulation of stomatal density influences stomatal size, plant growth and tolerance to restricted water supply across a growth carbon dioxide gradient. Philos. Trans. R. Soc. B 367, 547–555 (2012).
Masle, J., Gilmore, S. R. & Farquhar, G. D. The ERECTA gene regulates plant transpiration efficiency in Arabidopsis. Nature 436, 866–870 (2005).
Wang, Y., Hills, A. & Blatt, M. R. Systems analysis of guard cell membrane transport for enhanced stomatal dynamics and water use efficiency. Plant Physiol. 164, 1593–1599 (2014).
Kusumi, K., Hirotsuka, S., Kumamaru, T. & Iba, K. Increased leaf photosynthesis caused by elevated stomatal conductance in a rice mutant deficient in SLAC1, a guard cell anion channel protein. J. Exp. Bot. 63, 5635–5644 (2012).
Wang, Y. et al. Overexpression of plasma membrane H+-ATPase in guard cells promotes light-induced stomatal opening and enhances plant growth. Proc. Natl Acad. Sci. USA 111, 533–538 (2014).
Hecker, A. et al. Binary 2in1 vectors improve in planta (co-)localisation and dynamic protein interaction studies. Plant Physiol. 168, 776–787 (2015).
Grefen, C. et al. A novel motif essential for SNARE interaction with the K+ channel KC1 and channel gating in Arabidopsis. Plant Cell 22, 3076–3092 (2010).
Waghmare, S. et al. K+ channel–SEC11 binding exchange regulates SNARE assembly for secretory traffic. Plant Physiol. 181, 1096–1113 (2019).
Tyrrell, M. et al. Selective targeting of plasma membrane and tonoplast traffic by inhibitory (dominant-negative) SNARE fragments. Plant J. 51, 1099–1115 (2007).
Sani, E., Herzyk, P., Perrella, G., Colot, V. & Amtmann, A. Hyperosmotic priming of Arabidopsis seedlings establishes a long-term somatic memory accompanied by specific changes of the epigenome. Genome Biol. 14, R59 (2013).
Rasband, W. S. & Bright, D. S. NIH IMAGE—a public domain image-processing program for the Macintosh. Microbeam Anal. 4, 137–149 (1995).
Chen, Z. H., Hills, A., Lim, C. K. & Blatt, M. R. Dynamic regulation of guard cell anion channels by cytosolic free Ca2+ concentration and protein phosphorylation. Plant J. 61, 816–825 (2010).
Blatt, M. R. Electrical characteristics of stomatal guard cells: the contribution of ATP-dependent, ‘electrogenic’ transport revealed by current-voltage and difference-current-voltage analysis. J. Membr. Biol. 98, 257–274 (1987).
Hills, A., Chen, Z. H., Amtmann, A., Blatt, M. R. & Lew, V. L. OnGuard, a computational platform for quantitative kinetic modeling of guard cell physiology. Plant Physiol. 159, 1026–1042 (2012).
Chen, Z. H. et al. Systems dynamic modeling of the stomatal guard cell predicts emergent behaviors in transport, signaling, and volume control. Plant Physiol. 159, 1235–1251 (2012).
Jezek, M., Hills, A., Blatt, M. R. & Lew, V. L. A constraint–relaxation–recovery mechanism for stomatal dynamics. Plant, Cell Environ. 42, 2399–2410 (2019).
Karnik, R. et al. Arabidopsis Sec1/Munc18 protein SEC11 is a competitive and dynamic modulator of SNARE binding and SYP121-dependent vesicle traffic. Plant Cell 25, 1368–1382 (2013).
Clough, S. J. & Bent, A. F. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16, 735–743 (1998).
Acknowledgements
This work was supported by BBSRC grants BB/L001276/1, BB/L019205/1, BB/M001601/1 and BB/ T013508/1 to M.R.B., by a Royal Thai PhD studentship to W.H., a University of Glasgow Doctoral Training Studentship to W.C., a Lord Kelvin-Adam Smith Doctoral Fellowship to F.A.L.S.-A. and BBSRC grant BB/R019894/1 to A.A. We thank N. Donald for support in mbSUS assays and A. Ruiz-Pardo for help in plant maintenance.
Author information
Authors and Affiliations
Contributions
M.R.B. conceived the work and developed the strategies for analysis with B.Z., J.C.A. and A.H.; W.H., W.C. and B.Z. carried out and analysed the mbSUS assays; W.H. carried out oocyte electrophyiology and, with J.C.A., M.K. and M.R.B., the confocal, gas exchange and growth studies; M.K. carried out the guard cell electrophysiology and analysed the results with M.R.B.; S.W. expressed and purified the channel N-termini and carried out the gel filtration studies; M.P. and A.A. undertook the hydroponic growth studies; M.R.B. carried out the modelling with A.H., J.C.A., W.H., F.A.L.S.-A. and M.K.; M.R.B. wrote the manuscript, and all authors edited and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Plants thanks Anna Moroni, Xin-Guang Zhu, Nobuyuki Uozumi and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Tables 1–3, Figs. 1–15 and Appendix 1.
Source data
Source Data Fig. 3
Numerical data and statistics for Fig. 3b.
Source Data Fig. 4
Numerical data for Fig. 4a–f.
Source Data Fig. 5
Numerical data for Fig. 5a–c,e,f.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Horaruang, W., Klejchová, M., Carroll, W. et al. Engineering a K+ channel ‘sensory antenna’ enhances stomatal kinetics, water use efficiency and photosynthesis. Nat. Plants 8, 1262–1274 (2022). https://doi.org/10.1038/s41477-022-01255-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-022-01255-2