Abstract
Phloem transport of photoassimilates from leaves to non-photosynthetic organs, such as the root and shoot apices and reproductive organs, is crucial to plant growth and yield. For nearly 90 years, evidence has been generally consistent with the theory of a pressure-flow mechanism of phloem transport. Central to this hypothesis is the loading of osmolytes, principally sugars, into the phloem to generate the osmotic pressure that propels bulk flow. Here we used genetic and light manipulations to test whether sugar import into the phloem is required as the driving force for phloem sap flow. Using carbon-11 radiotracer, we show that a maize sucrose transporter1 (sut1) loss-of-function mutant has severely reduced export of carbon from photosynthetic leaves (only ~4% of the wild type level). Yet, the mutant remarkably maintains phloem pressure at ~100% and sap flow speeds at ~50–75% of those of wild type. Potassium (K+) abundance in the phloem was elevated in sut1 mutant leaves. Fluid dynamic modelling supports the conclusion that increased K+ loading compensated for decreased sucrose loading to maintain phloem pressure, and thereby maintained phloem transport via the pressure-flow mechanism. Furthermore, these results suggest that sap flow and transport of other phloem-mobile nutrients and signalling molecules could be regulated independently of sugar loading into the phloem, potentially influencing carbon–nutrient homoeostasis and the distribution of signalling molecules in plants encountering different environmental conditions.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All of the data supporting the findings of this study are included within the article and the accompanying supplementary material. Source data are provided with this paper.
Availability of materials
Zea mays sut1 loss-of-function mutant seed may be obtained from the Maize Genetics Stock Center (http://maizecoop.cropsci.uiuc.edu/). Source data are provided with this paper.
References
Braun, D. M., Wang, L. & Ruan, Y.-L. Understanding and manipulating sucrose phloem loading, unloading, metabolism, and signalling to enhance crop yield and food security. J. Exp. Bot. 65, 1713–1735 (2014).
Julius, B. T., Leach, K. A., Tran, T. M., Mertz, R. A. & Braun, D. M. Sugar transporters in plants: new insights and discoveries. Plant Cell Physiol. 58, 1442–1460 (2017).
Slewinski, T. L. & Braun, D. M. Current perspectives on the regulation of whole-plant carbohydrate partitioning. Plant Sci. 178, 341–349 (2010).
Smith, A. M. & Stitt, M. Coordination of carbon supply and plant growth. Plant Cell Environ. 30, 1126–1149 (2007).
Sulpice, R. et al. Starch as a major integrator in the regulation of plant growth. Proc. Natl Acad. Sci. USA 106, 10348–10353 (2009).
Sulpice, R. et al. Network analysis of enzyme activities and metabolite levels and their relationship to biomass in a large panel of Arabidopsis accessions. Plant Cell 22, 2872–2893 (2010).
Mason, M. G., Ross, J. J., Babst, B. A., Wienclaw, B. N. & Beveridge, C. A. Sugar demand, not auxin, is the initial regulator of apical dominance. Proc. Natl Acad. Sci. USA 111, 6092–6097 (2014).
Knoblauch, M. & Peters, W. S. Munch, morphology, microfluidics - our structural problem with the phloem. Plant Cell Environ. 33, 1439–1452 (2010).
Münch, E. Die Stoffbewegungen in der Pflanze (Gustav Fischer, 1930).
Ayre, B. G. Membrane-transport systems for sucrose in relation to whole-plant carbon partitioning. Mol. Plant 4, 377–394 (2011).
Turgeon, R. The puzzle of phloem pressure. Plant Physiol. 154, 578–581 (2010).
Giaquinta, R. T. Phloem loading of sucrose. Annu. Rev. Plant Physiol. 34, 347–387 (1983).
Patrick, J. W., Zhang, W., Tyerman, S. D., Offler, C. E. & Walker, N. A. Role of membrane transport in phloem translocation of assimilates and water. Aust. J. Plant Physiol. 28, 695–707 (2001).
Rennie, E. A. & Turgeon, R. A comprehensive picture of phloem loading strategies. Proc. Natl Acad. Sci. USA 106, 14162–14167 (2009).
van Bel, A. J. E. & Gamalei, Y. V. Ecophysiology of phloem loading in source leaves. Plant Cell Environ. 15, 265–270 (1992).
Gould, N., Thorpe, M. R., Koroleva, O. & Minchin, P. E. H. Phloem hydrostatic pressure relates to solute loading rate: a direct test of the Münch hypothesis. Funct. Plant Biol. 32, 1019–1026 (2005).
Knoblauch, M. et al. Testing the Münch hypothesis of long distance phloem transport in plants. eLife 5, e15341 (2016).
Patrick, J. W. Does Don Fisher’s high-pressure manifold model account for phloem transport and resource partitioning? Front. Plant Sci. 4, 184 (2013).
Bürkle, L. et al. The H+-sucrose cotransporter NtSUT1 is essential for sugar export from tobacco leaves. Plant Physiol. 118, 59–68 (1998).
Gottwald, J. R., Krysan, P. J., Young, J. C., Evert, R. F. & Sussman, M. R. Genetic evidence for the in planta role of phloem-specific plasma membrane sucrose transporters. Proc. Natl Acad. Sci. USA 97, 13979–13984 (2000).
Riesmeier, J. W., Willmitzer, L. & Frommer, W. B. Evidence for an essential role of the sucrose transporter in phloem loading and assimilate partitioning. EMBO J. 13, 1–7 (1994).
Slewinski, T. L., Meeley, R. & Braun, D. M. Sucrose transporter1 functions in phloem loading in maize leaves. J. Exp. Bot. 60, 881–892 (2009).
Srivastava, A. C., Ganesan, S., Ismail, I. O. & Ayre, B. G. Functional characterization of the Arabidopsis AtSUC2 sucrose/H+ symporter by tissue-specific complementation reveals an essential role in phloem loading but not in long-distance transport. Plant Physiol. 148, 200–211 (2008).
Gamalei, Y. V. Structure and function of leaf minor veins in trees and herbs. A taxonomic review. Trees 3, 96–110 (1989).
Turgeon, R. The role of phloem loading reconsidered. Plant Physiol. 152, 1817–1823 (2010).
Turgeon, R. & Medville, R. The absence of phloem loading in willow leaves. Proc. Natl Acad. Sci. USA 95, 12055–12060 (1998).
Zhang, C. et al. Symplastic phloem loading in poplar. Plant Physiol. 166, 306–313 (2014).
Slewinski, T. L., Zhang, C. & Turgeon, R. Structural and functional heterogeneity in phloem loading and transport. Front. Plant Sci. 4, 244 (2013).
Braun, D. M. & Slewinski, T. L. Genetic control of carbon partitioning in grasses: roles of sucrose transporters and tie-dyed loci in phloem loading. Plant Physiol. 149, 71–81 (2009).
Evert, R. F., Eschrich, W. & Heyser, W. Leaf structure in relation to solute transport and phloem loading. Planta 138, 279–294 (1978).
Babst, B. A., Karve, A. A. & Judt, T. Radio-metabolite analysis of carbon-11 biochemical partitioning to nonstructural carbohydrates for integrated metabolism and transport studies. Plant Cell Physiol. 54, 1016–1025 (2013).
Lohaus, G. et al. Solute balance of a maize (Zea mays L.) source leaf as affected by salt treatment with special emphasis on phloem retranslocation and ion leaching. J. Exp. Bot. 51, 1721–1732 (2000).
Ohshima, T., Hayashi, H. & Chino, M. Collection and chemical composition of pure phloem sap from Zea mays L. Plant Cell Physiol. 31, 735–737 (1990).
Weiner, H., Blechschmidt-Schneider, S., Mohme, H., Eschrich, W. & Heldt, H. W. Phloem transport of amino-acids. Comparison of amino-acid contents of maize leaves and of the sieve tube exudate. Plant Physiol. Biochem. 29, 19–23 (1991).
Baker, R. F. et al. Sucrose transporter ZmSut1 expression and localization uncover new insights into sucrose phloem loading. Plant Physiol. 172, 1876–1898 (2016).
Gould, N. et al. AtSUC2 has a role for sucrose retrieval along the phloem pathway: evidence from carbon-11 tracer studies. Plant Sci. 188-189, 97–101 (2012).
Knoblauch, J., Mullendore, D. L., Jensen, K. H. & Knoblauch, M. Pico gauges for minimally invasive intracellular hydrostatic pressure measurements. Plant Physiol. 166, 1271–1279 (2014).
Babst, B. A. et al. Jasmonic acid induces rapid changes in carbon transport and partitioning in Populus. New Phytol. 167, 63–72 (2005).
Tran, T. M. et al. In vivo transport of three radioactive [18F]-fluorinated deoxysucrose analogs by the maize sucrose transporter ZmSUT1. Plant Physiol. Biochem. 115, 1–11 (2017).
Jensen, K. H., Rio, E., Hansen, R., Clanet, C. & Bohr, T. Osmotically driven pipe flows and their relation to sugar transport in plants. J. Fluid Mech. 636, 371–396 (2009).
Hanik, N. et al. Partitioning of new carbon as 11C in Nicotiana tabacum reveals new insight into methyl jasmonate induced changes in metabolism. J. Chem. Ecol. 36, 1058–1067 (2010).
Hewer, A., Becker, A. & van Bel, A. J. E. An aphid’s Odyssey - the cortical quest for the vascular bundle. J. Exp. Biol. 214, 3868–3879 (2011).
Hewer, A., Will, T. & van Bel, A. J. E. Plant cues for aphid navigation in vascular tissues. J. Exp. Biol. 213, 4030–4042 (2010).
Pescod, K. V., Quick, W. P. & Douglas, A. E. Aphid responses to plants with genetically manipulated phloem nutrient levels. Physiol. Entomol. 32, 253–258 (2007).
De Vos, M. et al. Signal signature and transcriptome changes of Arabidopsis during pathogen and insect attack. Mol. Plant Microbe Interact. 18, 923–937 (2005).
Cherqui, A. & Tjallingii, W. F. Salivary proteins of aphids, a pilot study on identification, separation and immunolocalisation. J. Insect Physiol. 46, 1177–1186 (2000).
Miles, P. W. Aphid saliva. Biol. Rev. 74, 41–85 (1999).
Will, T., Tjallingii, W. F., Thönnessen, A. & van Bel, A. J. E. Molecular sabotage of plant defense by aphid saliva. Proc. Natl Acad. Sci. USA 104, 10536–10541 (2007).
Kopittke, P. M. et al. Synchrotron-based X-ray fluorescence microscopy as a technique for imaging of elements in plants. Plant Physiol. 178, 507–523 (2018).
Punshon, T., Guerinot, M. L. & Lanzirotti, A. Using synchrotron X-ray fluorescence microprobes in the study of metal homeostasis in plants. Ann. Bot. 103, 665–672 (2009).
Punshon, T. et al. The role of CAX1 and CAX3 in elemental distribution and abundance in Arabidopsis seed. Plant Physiol. 158, 352–362 (2012).
Sarret, G. et al. in Advances in Agronomy Vol. 119 (ed. Sparks, D. L.) 1–82 (Academic Press, 2013).
Comtet, J., Jensen, K. H., Turgeon, R., Stroock, A. D. & Hosoi, A. E. Passive phloem loading and long-distance transport in a synthetic tree-on-a-chip. Nat. Plants 3, 17032 (2017).
Jensen, K. H., Liesche, J., Bohr, T. & Schulz, A. Universality of phloem transport in seed plants. Plant Cell Environ. 35, 1065–1076 (2012).
Slewinski, T. L., Garg, A., Johal, G. S. & Braun, D. M. Maize SUT1 functions in phloem loading. Plant Signal. Behav. 5, 687–690 (2010).
Babst, B. A., Ferrieri, R. A., Thorpe, M. R. & Orians, C. M. Lymantria dispar herbivory induces rapid changes in carbon transport and partitioning in Populus nigra. Entomol. Exp. Appl. 128, 117–125 (2008).
Karve, A. et al. In vivo quantitative imaging of photoassimilate transport dynamics and allocation in large plants using a commercial positron emission tomography (PET) scanner. BMC Plant Biol. 15, 273 (2015).
Keutgen, A. J. et al. Input–output analysis of in vivo photoassimilate translocation using Positron-Emitting Tracer Imaging System (PETIS) data. J. Exp. Bot. 56, 1419–1425 (2005).
Kikuchi, K. et al. Real-time analysis of photoassimilate translocation in intact eggplant fruit using 11CO2 and a positron-emitting tracer imaging system. J. Jpn. Soc. Hortic. Sci. 77, 199–205 (2008).
Moorby, J., Ebert, M. & Evans, N. T. S. The translocation of 11C-labelled photosynthate in the soybean. J. Exp. Bot. 14, 210–220 (1963).
Moorby, J., Troughton, J. H. & Currie, B. G. Investigations of carbon transport in plants. II. The effects of light and darkness and sink activity on translocation. J. Exp. Bot. 25, 937–944 (1974).
Troughton, J. H., Currie, B. G. & Chang, F. H. Relations between light level, sucrose concentration, and translocation of carbon 11 in Zea mays leaves. Plant Physiol. 59, 808–820 (1977).
Smith, J. A. C. & Milburn, J. A. Osmoregulation and the control of phloem-sap composition in Ricinus communis L. Planta 148, 28–34 (1980).
Deeken, R. et al. Loss of the AKT2/3 potassium channel affects sugar loading into the phloem of Arabidopsis. Planta 216, 334–344 (2002).
Philippar, K. et al. The K+ channel KZM1 mediates potassium uptake into the phloem and guard cells of the C4 grass Zea mays. J. Biol. Chem. 278, 16973–16981 (2003).
Dreyer, I., Gomez-Porras, J. L. & Riedelsberger, J. The potassium battery: a mobile energy source for transport processes in plant vascular tissues. New Phytol. 216, 1049–1053 (2017).
Gajdanowicz, P. et al. Potassium (K+) gradients serve as a mobile energy source in plant vascular tissues. Proc. Natl Acad. Sci. USA 108, 864–869 (2011).
Fu, Q., Cheng, L., Guo, Y. & Turgeon, R. Phloem loading strategies and water relations in trees and herbaceous plants. Plant Physiol. 157, 1518–1527 (2011).
Ham, B.-K. & Lucas, W. J. Phloem-mobile RNAs as systemic signaling agents. Annu. Rev. Plant Biol. 68, 173–195 (2017).
Jung, H. W., Tschaplinski, T. J., Wang, L., Glazebrook, J. & Greenberg, J. T. Priming in systemic plant immunity. Science 324, 89–91 (2009).
Kachroo, A. & Robin, G. P. Systemic signaling during plant defense. Curr. Opin. Plant Biol. 16, 527–533 (2013).
Lucas, W. J. et al. The plant vascular system: evolution, development and functions. J. Integr. Plant Biol. 55, 294–388 (2013).
Ohkubo, Y., Tanaka, M., Tabata, R., Ogawa-Ohnishi, M. & Matsubayashi, Y. Shoot-to-root mobile polypeptides involved in systemic regulation of nitrogen acquisition. Nat. Plants 3, 17029 (2017).
Park, S.-W., Kaimoyo, E., Kumar, D., Mosher, S. & Klessig, D. F. Methyl salicylate is a critical mobile signal for plant systemic acquired resistance. Science 318, 113–116 (2007).
Sattar, S. & Thompson, G. A. Small RNA regulators of plant–hemipteran interactions: micromanagers with versatile roles. Front. Plant Sci. 7, 1241 (2016).
Shine, M. B., Xiao, X., Kachroo, P. & Kachroo, A. Signaling mechanisms underlying systemic acquired resistance to microbial pathogens. Plant Sci. 279, 81–86 (2018).
Drobnitch, S. T., Jensen, K. H., Prentice, P. & Pittermann, J. Convergent evolution of vascular optimization in kelp (Laminariales). Proc. R. Soc. B 282, 20151667 (2015).
Knoblauch, J., Drobnitch, S. T., Peters, W. S. & Knoblauch, M. In situ microscopy reveals reversible cell wall swelling in kelp sieve tubes: one mechanism for turgor generation and flow control? Plant Cell Environ. 39, 1727–1736 (2016).
Knoblauch, J., Peters, W. S. & Knoblauch, M. The gelatinous extracellular matrix facilitates transport studies in kelp: visualization of pressure-induced flow reversal across sieve plates. Ann. Bot. 117, 599–606 (2016).
Raven, J. A. Polar auxin transport in relation to long-distance transport of nutrients in the Charales. J. Exp. Bot. 64, 1–9 (2013).
Rotsch, D. et al. Radiosynthesis of 6’-deoxy-6’[18F]fluorosucrose via automated synthesis and its utility to study in vivo sucrose transport in maize (Zea mays) leaves. PLoS ONE 10, e0128989 (2015).
Ma, Y., Slewinski, T. L., Baker, R. F. & Braun, D. M. Tie-dyed1 encodes a novel, phloem-expressed transmembrane protein that functions in carbohydrate partitioning. Plant Physiol. 149, 181–194 (2009).
Bihmidine, S., Baker, R. F., Hoffner, C. & Braun, D. M. Sucrose accumulation in sweet sorghum stems occurs by apoplasmic phloem unloading and does not involve differential sucrose transporter expression. BMC Plant Biol. 15, 1–22 (2015).
Mullendore, D. L., Windt, C. W., Van As, H. & Knoblauch, M. Sieve tube geometry in relation to phloem flow. Plant Cell 22, 579–593 (2010).
Nadwodnik, J. & Lohaus, G. Subcellular concentrations of sugar alcohols and sugars in relation to phloem translocation in Plantago major, Plantago maritima, Prunus persica, and Apium graveolens. Planta 227, 1079–1089 (2008).
Acknowledgements
This work was supported in part by: the United States Department of Energy, Office of Biological and Environmental Research (contract DE-AC02-98CH10886); a Goldhaber Distinguished Fellowship to B.A.B.; the United States Department of Agriculture, National Institute of Food and Agriculture, McEntire-Stennis project number 1009319 (B.A.B.); a US National Science Foundation Plant Genome Research Program grant (IOS-1025976) to D.M.B.; research grants from Villum Fonden (37475) and the Independent Research Fund Denmark (9064-00069B) to K.H.J.; and a DFG grant to R.H. and S.S. (SCHE 2148/1-1). This research used the X27A Beamline of the National Synchrotron Light Source, a US Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Brookhaven National Laboratory under Contract No. DE- AC02-98CH10886.
Author information
Authors and Affiliations
Contributions
B.A.B., A.A.K., D.J.K. and J.R. performed 11C radiotracer experiments. R.F.B. and T.M.T. performed genotyping by PCR, vein counts and carboxyfluorescein experiments. J.K. performed pico-gauge measurements of sieve tube pressures and determination of phloem conductivity. B.A.B. and D.M.B. designed initial 11C experiments. B.A.B., D.M.B., J.K., M.K., K.H.J., R.H. and S.S. designed subsequent experiments. B.A.B. and R.T. performed synchrotron X-ray fluorescence measurements on frozen hydrated tissue and R.T. performed image analysis and quantification. G.L. performed aphid stylectomy and analysis of phloem sap. S.S. and R.H. conducted K+ microelectrode experiments. K.H.J. completed the model of sugar transport, with input from B.A.B., J.K. and M.K. B.A.B. drafted the manuscript with input and editing from all co-authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Plants thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
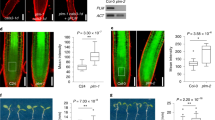
Extended Data Fig. 1 Feeding of 11CO2 to a leaf.
Photograph showing Carbon-11 (11C) being administered as 11CO2 to a single leaf of a maize plant, clamped in a leaf cuvette. There was a single radiation detector built into the leaf cuvette, and two other detectors positioned along the leaf, basipetal to the leaf cuvette.
Extended Data Fig. 2 Homozygous sut1 mutant leaves had high carbohydrate concentrations, and low carbohydrate specific activity.
Analysis of leaf extracts for (a) unlabeled (12C) soluble sucrose (Poverall model = 0.0001; Pwt-vs-sut1 = 0.0003; Phet-vs-sut1 = 0.0003; Pwt-vs-het = 1.0), glucose (Poverall model = 0.012; Pwt-vs-sut1 = 0.015; Phet-vs-sut1 = 0.036; Pwt-vs-het = 0.80), fructose (Poverall model < 0.0001; Pwt-vs-sut1 = 0.0002; Phet-vs-sut1 = 0.0002; Pwt-vs-het = 1.0), and starch concentrations (Poverall model = 0.003; Pwt-vs-sut1 = 0.008; Phet-vs-sut1 = 0.005; Pwt-vs-het = 0.92), (b) partitioning of 11C to soluble sucrose (Poverall model = 0.0014; Pwt-vs-sut1 = 0.002; Phet-vs-sut1 = 0.004; Pwt-vs-het = 0.92), glucose (Poverall model = 0.009; Pwt-vs-sut1 = 0.015; Phet-vs-sut1 = 0.02; Pwt-vs-het = 0.97), fructose (Poverall model = 0.054), and starch (Poverall model = 0.16) as a percentage of the total fixed 11C, and (c) specific activity of sucrose (Poverall model = 0.0159; Pwt-vs-sut1 = 0.059; Phet-vs-sut1 = 0.017; Pwt-vs-het = 0.69), glucose (Poverall model = 0.23), fructose (Poverall model = 0.014; Pwt-vs-sut1 = 0.017; Phet-vs-sut1 = 0.043; Pwt-vs-het = 0.79), and starch (Poverall model = 0.022; Pwt-vs-sut1 = 0.66; Phet-vs-sut1 = 0.02; Pwt-vs-het = 0.08) shown as GBq of 11C radioactivity per gram of the sugar or starch, respectively (WT n = 3, het n = 3, sut1 n = 4). Specific activity indicates the proportion of 11C to 12C. Biochemical partitioning of 11C to soluble sugars but not starch was elevated in sut1 mutant leaves. The central line is the median, the box indicates the first and third quartiles, and the whiskers indicate the minimum and maximum values. Statistical significance according to a one-way ANOVA is indicated by *(P<0.05), **(P<0.01), ***(P<0.001), or NS (not significant). Different letters above bars indicate which genotypes were statistically different based on a Tukey’s post hoc test.
Extended Data Fig. 3 When 11C dilution was mitigated by reducing sugar concentrations in sut1 mutant leaves by dark treatment, 11C export remained extremely low in sut1 mutants, but sap flow velocity remained high by comparison.
Because the concentration of [12C]-sucrose was extremely high in sut1 leaves, and the specific activity was low (Fig. 1F and H, respectively; Extended Data Fig. 2), one could speculate that the reduced 11C export in sut1 might have appeared more drastic than overall 12C export due to isotopic dilution. However, it is possible that much of the sucrose was unavailable for phloem loading in sut1 mutants, for example if much of it is stored in vacuoles or exuded from hydathodes44,74,75. To measure export under more uniform cellular sugar status, we moved sut1 mutant plants into constant darkness for 42 hrs immediately prior to 11C assays, reducing the unlabeled (12C) leaf sucrose, glucose, fructose and starch concentrations (a) to near WT levels. Note the 30 fold difference in Y-axis scaling for sugar concentrations compared to Extended Data Fig. 2a (6 fold for starch). (b) 11C-labeled sucrose (Poverall model <0.0001; Pwt-vs-sut1 <0.0001; Phet-vs-sut1 = 0.0002; Pwt-vs-het = 0.89), glucose (Poverall model = 0.0051; Pwt-vs-sut1 = 0.0056; Phet-vs-sut1 = 0.042; Pwt-vs-het = 0.61), fructose (Poverall model = 0.017; Pwt-vs-sut1 = 0.018; Phet-vs-sut1 = 0.091; Pwt-vs-het = 0.71), and starch. (c) Specific activities of sucrose, glucose, fructose, and starch were similar in WT and sut1 mutant plants after dark treatment. For all panels, the central line is the median, the box indicates the first and third quartiles, and the whiskers indicate the minimum and maximum values (WT n = 5, het n = 4, and sut1 n = 7). Statistical significance according to a one-way ANOVA is indicated by *(P<0.05), **(P<0.01), ***(P<0.001). Different letters above bars in indicate which genotypes were statistically different based on a Tukey’s post hoc test. In these conditions, we confirmed that the greatly reduced 11C-export by sut1 mutant leaves (Figs. 1c and 2b) was not an artifact of isotopic dilution by high [12C]-sucrose concentrations. The reduction in phloem sap flow velocities in the low-carbohydrate, dark-treated sut1 mutant leaves relative to WT remained modest in this experiment (Fig. 2c), compared to the large reduction of 11C-photoassimilate export (Fig. 2b), similar to the experiment with no dark treatment (Fig. 1c-d).
Extended Data Fig. 4 Maize phloem sap contents did not significantly correlate with phloem sucrose content.
Phloem sap was collected from wild type (blue diamonds) and sut1 mutant (yellow circles) leaves by aphid stylectomy. We tested for a negative correlation between sucrose and (a) potassium, (b) chloride, (c) sodium, (d) total amino acids, (e) magnesium, (f) calcium, (g) total cations, and (h) potassium, sodium, and chloride combined (See Table 1 for P values).
Extended Data Fig. 5 Phloem sap potassium (K+) concentrations were higher in sut1 mutant leaves than in wild type leaves.
(a) Comparison of wild type (WT) and mutant (sut1) leaf phloem. The central line is the median, and the box and whiskers indicate the first and third quartiles, and the the minimum and maximum values, respectively (n = 13, *** indicates P = 0.0003 for a two-sided Student’s t-test). (b) K+-selective microprobes were calibrated using K+ standard solutions of known concentration. The reference electrode (blue) was hardly affected by changing external K+ concentrations (concentration shown below the graph ranging from 1M to 1 mM), while the K+- selective electrode (red) shows a near Nernstian behavior. Thus, the reference subtracted black line shifts with the given values of K+ indicating a fast and functional K+-selective setup (values used for calibration are given below the black line). (c) Microscopic image of K+-selective electrode (upper) and reference electrode (lower) impaled into a phloem cell. Both electrodes were controlled by micromanipulators under a microscope for impalement, and both electrodes were calibrated before and after impalement. (d) Raw trace of an example measurement before and after impalement (blue arrow indicates time of reference electrode impalement; red arrow indicates timing of K+-selective electrode impalement). Black trace shows the membrane potential (blue trace) subtracted from the K+-selective potential (red trace). We measured the stable selective potential (grey area) for all plants before removing the selective electrode at the end of the measurement.
Extended Data Fig. 6 No effect of potassium supplementation on 11C-photoassimilate transport dynamics.
Potassium supplementation of the soil medium did not affect 11C export or transport dynamics in either wild type (WT) or sut1 mutant leaves. (a) Severely reduced 11C export rates from sut1 homozygous mutant leaves. (b) Marginally reduced sap flow velocity of 11C-photoassimilates in sut1 mutant leaves was not alleviated by adding 200 mL of 20 mM KCl to each pot once per week, starting 2 weeks after sowing. HK, high potassium; LK, low potassium. For both panels, center line is median, boxes indicate first and third quartiles, and whiskers are minimum and maximum (n = 4 for all bars, except for sap flow velocity for sut1 low K+, which was n = 3). For export (a), the overall model was significant for a two-way ANOVA (P <0.0001), and effects of genotype were significant (P <0.0001), but there were no statistically significant effects of K+ treatment (P = 0.40) or interactions between genotype and potassium treatment (P = 0.53). For sap flow velocity (b), the overall model was not significant for a two-way ANOVA (P = 0.16), the effects of genotype were significant (P = 0.034), but there were no statistically significant effects of K+ treatment (P = 0.84) or interactions between genotype and potassium treatment (P = 0.55). Statistical significance of genotype effects are indicated by *(P<0.05), **(P<0.01), ***(P<0.001).
Supplementary information
Supplementary Information
Supplementary Tables 1 and 2,. and text.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Rights and permissions
About this article
Cite this article
Babst, B.A., Braun, D.M., Karve, A.A. et al. Sugar loading is not required for phloem sap flow in maize plants. Nat. Plants 8, 171–180 (2022). https://doi.org/10.1038/s41477-022-01098-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-022-01098-x
This article is cited by
-
Communication between plant roots and the soil microbiome; involvement in plant growth and development
Symbiosis (2023)
-
Low sugar, under pressure?
Nature Plants (2022)
-
Genomic Analyses of SUT and TST Sugar Transporter Families in Low and High Sugar Accumulating Sugarcane Species (Saccharum spontaneum and Saccharum officinarum)
Tropical Plant Biology (2022)