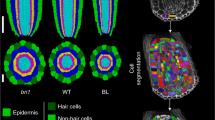
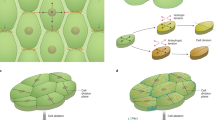
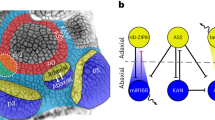
Abstract
Plants generate a large variety of shoot forms with regular geometries. These forms emerge primarily from the activity of a stem cell niche at the shoot tip. Recent efforts have established a theoretical framework of form emergence at the shoot tip, which has empowered the use of modelling in conjunction with biological approaches to begin to disentangle the biochemical and physical mechanisms controlling form development at the shoot tip. Here, we discuss how these advances get us closer to identifying the construction principles of plant shoot tips. Considering the current limits of our knowledge, we propose a roadmap for developing a general theory of form development at the shoot tip.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Turing, A. M. The chemical basis of morphogenesis. Proc. R. Soc. Lond. B 237, 37–72 (1952).
Jaeger, J., Irons, D. & Monk, N. Regulative feedback in pattern formation: towards a general relativistic theory of positional information. Development 135, 3175–3183 (2008).
Adler, I., Barabe, D. & Jean, R. V. A history of the study of phyllotaxis. Ann. Bot. 80, 231–244 (1997).
Douady, S. & Couder, Y. Phyllotaxis as a dynamical self-organizing process. 2. The spontaneous formation of a periodicity and the coexistence of spiral and whorled patterns. J. Theor. Biol. 178, 275–294 (1996).
Refahi, Y. et al. A stochastic multicellular model identifies biological watermarks from disorders in self-organized patterns of phyllotaxis. eLife 5, e14093 (2016).
Godin, C., Golé, C. & Douady, S. Phyllotaxis as geometric canalization during plant development. Development 147, dev165878 (2020).
Sachs, J. A Text-Book of Botany: Morphological and Physiological (Clarendon Press, 1875).
Kutschera, U. in The Cytoskeletal Basis of Plant Growth and Form (ed. Lloyd, C. W.) 85–99 (Academic Press, 1991).
Lockhart, J. A. An analysis of irreversible plant cell elongation. J. Theor. Biol. 8, 264–275 (1965).
Ray, P. M., Green, P. B. & Cleland, R. Role of turgor in plant cell growth. Nature 239, 163–164 (1972).
Dupuy, L., Mackenzie, J., Rudge, T. & Haseloff, J. A system for modelling cell-cell interactions during plant morphogenesis. Ann. Bot. 101, 1255–1265 (2008).
Hamant, O. et al. Developmental patterning by mechanical signals in Arabidopsis. Science 322, 1650–1655 (2008).
Boudon, F. et al. A computational framework for 3D mechanical modeling of plant morphogenesis with cellular resolution. PLoS Comput. Biol. 11, e1003950 (2015).
Bozorg, B., Krupinski, P. & Jönsson, H. A continuous growth model for plant tissue. Phys. Biol. 13, 065002 (2016).
Erickson, R. O. Modeling of plant growth. Annu. Rev. Plant Physiol. 27, 407–434 (1976).
Silk, W. K. Quantitative descriptions of development. Annu. Rev. Plant Physiol. 35, 479–518 (1984).
Coen, E., Rolland-Lagan, A.-G., Matthews, M., Bangham, J. A. & Prusinkiewicz, P. The genetics of geometry. Proc. Natl Acad. Sci. USA 101, 4728–4735 (2004).
Abley, K. et al. An intracellular partitioning-based framework for tissue cell polarity in plants and animals. Development 140, 2061–2074 (2013).
Bassel, G. W. et al. Mechanical constraints imposed by 3D cellular geometry and arrangement modulate growth patterns in the Arabidopsis embryo. Proc. Natl Acad. Sci. USA 111, 8685–8690 (2014).
Bozorg, B., Krupinski, P. & Jönsson, H. Stress and strain provide positional and directional cues in development. PLoS Comput. Biol. 10, e1003410 (2014).
Oliveri, H., Traas, J., Godin, C. & Ali, O. Regulation of plant cell wall stiffness by mechanical stress: a mesoscale physical model. J. Math. Biol. 78, 625–653 (2019).
Gaillochet, C., Daum, G. & Lohmann, J. U. O cell, where art thou? The mechanisms of shoot meristem patterning. Curr. Opin. Plant Biol. 23, 91–97 (2015).
Jönsson, H. et al. Modeling the organization of the WUSCHEL expression domain in the shoot apical meristem. Bioinformatics 21, i232–i240 (2005).
Hohm, T., Zitzler, E. & Simon, R. A dynamic model for stem cell homeostasis and patterning in Arabidopsis meristems. PLoS ONE 5, e9189 (2010).
Yadav, R. K. et al. Plant stem cell maintenance involves direct transcriptional repression of differentiation program. Mol. Syst. Biol. 9, 654 (2013).
Gruel, J. et al. An epidermis-driven mechanism positions and scales stem cell niches in plants. Sci. Adv. 2, e1500989 (2016).
Adibi, M., Yoshida, S., Weijers, D. & Fleck, C. Centering the organizing center in the Arabidopsis thaliana shoot apical meristem by a combination of cytokinin signaling and self-organization. PLoS ONE 11, e0147830 (2016).
Zhou, Y. et al. HAIRY MERISTEM with WUSCHEL confines CLAVATA3 expression to the outer apical meristem layers. Science 361, 502–506 (2018).
Gruel, J., Deichmann, J., Landrein, B., Hitchcock, T. & Jönsson, H. The interaction of transcription factors controls the spatial layout of plant aerial stem cell niches. npj Syst. Biol. Appl. 4, 36 (2018).
Knauer, S. et al. A protodermal miR394 signal defines a region of stem cell competence in the Arabidopsis shoot meristem. Dev. Cell 24, 125–132 (2013).
Benkova, E., Ivanchenko, M. G., Friml, J., Shishkova, S. & Dubrovsky, J. G. A morphogenetic trigger: is there an emerging concept in plant developmental biology? Trends Plant Sci. 14, 189–193 (2009).
Paque, S. & Weijers, D. Auxin: the plant molecule that influences almost anything. BMC Biol. 14, 675 (2016).
Vernoux, T. et al. The auxin signalling network translates dynamic input into robust patterning at the shoot apex. Mol. Syst. Biol. 7, 508–508 (2011).
de Reuille, P. B. et al. Computer simulations reveal properties of the cell-cell signaling network at the shoot apex in Arabidopsis. Proc. Natl Acad. Sci. USA 103, 1627–1632 (2006).
Galvan-Ampudia, C. S. et al. Temporal integration of auxin information for the regulation of patterning. eLife 9, e55832 (2020).
Reinhardt, D., Mandel, T. & Kuhlemeier, C. Auxin regulates the initiation and radial position of plant lateral organs. Plant Cell 12, 507–518 (2000).
Zhao, Z. et al. Hormonal control of the shoot stem-cell niche. Nature 465, 1089–1092 (2010).
Ma, Y. et al. WUSCHEL acts as an auxin response rheostat to maintain apical stem cells in Arabidopsis. Nat. Commun. 10, 5093 (2019).
Truskina, J. et al. A network of transcriptional repressors modulates auxin responses. Nature 589, 116–119 (2021).
Vernoux, T., Kronenberger, J., Grandjean, O., Laufs, P. & Traas, J. PIN-FORMED 1 regulates cell fate at the periphery of the shoot apical meristem. Development 127, 5157–5165 (2000).
Reinhardt, D. et al. Regulation of phyllotaxis by polar auxin transport. Nature 426, 255–260 (2003).
Heisler, M. G. et al. Patterns of auxin transport and gene expression during primordium development revealed by live imaging of the Arabidopsis inflorescence meristem. Curr. Biol. 15, 1899–1911 (2005).
Bainbridge, K. et al. Auxin influx carriers stabilize phyllotactic patterning. Genes Dev. 22, 810–823 (2008).
Jönsson, H., Heisler, M. G., Shapiro, B. E., Meyerowitz, E. M. & Mjolsness, E. An auxin-driven polarized transport model for phyllotaxis. Proc. Natl Acad. Sci. USA 103, 1633–1638 (2006).
Smith, R. S. et al. A plausible model of phyllotaxis. Proc. Natl Acad. Sci. USA 103, 1301–1306 (2006).
Stoma, S. et al. Flux-based transport enhancement as a plausible unifying mechanism for auxin transport in meristem development. PLoS Comput. Biol. 4, e1000207 (2008).
Bayer, E. M. et al. Integration of transport-based models for phyllotaxis and midvein formation. Genes Dev. 23, 373–384 (2009).
van Berkel, K., de Boer, R. J., Scheres, B. & Tusscher ten, K. Polar auxin transport: models and mechanisms. Development 140, 2253–2268 (2013).
O’Connor, D. L. et al. A division in PIN-mediated auxin patterning during organ initiation in grasses. PLoS Comput. Biol. 10, e1003447 (2014).
Hartmann, F. P., Barbier de Reuille, P. & Kuhlemeier, C. Toward a 3D model of phyllotaxis based on a biochemically plausible auxin-transport mechanism. PLoS Comput. Biol. 15, e1006896 (2019).
Brunoud, G. et al. A novel sensor to map auxin response and distribution at high spatio-temporal resolution. Nature 482, 103–106 (2012).
Abley, K., Sauret-Güeto, S., Marée, A. F. & Coen, E. Formation of polarity convergences underlying shoot outgrowths. eLife 5, e18165 (2016).
Caggiano, M. P. et al. Cell type boundaries organize plant development. eLife 6, e27421 (2017).
Besnard, F. et al. Cytokinin signalling inhibitory fields provide robustness to phyllotaxis. Nature 505, 417–421 (2014).
Shi, B. et al. Feedback from lateral organs controls shoot apical meristem growth by modulating auxin transport. Dev. Cell 44, 204–216 (2018).
Baskin, T. I. Anisotropic expansion of the plant cell wall. Annu. Rev. Cell Dev. Biol. 21, 203–222 (2005).
Corson, F. et al. Turning a plant tissue into a living cell froth through isotropic growth. Proc. Natl Acad. Sci. USA 106, 8453–8458 (2009).
Landrein, B. & Hamant, O. How mechanical stress controls microtubule behavior and morphogenesis in plants: history, experiments and revisited theories. Plant J. 75, 324–338 (2013).
Sampathkumar, A. et al. Primary wall cellulose synthase regulates shoot apical meristem mechanics and growth. Development 146, dev179036 (2019).
Sassi, M. et al. An auxin-mediated shift toward growth isotropy promotes organ formation at the shoot meristem in Arabidopsis. Curr. Biol. 24, 2335–2342 (2014).
Armezzani, A. et al. Transcriptional induction of cell wall remodelling genes is coupled to microtubule-driven growth isotropy at the shoot apex in Arabidopsis. Development 145, dev162255 (2018).
Fleming, A. J., McQueen-Mason, S. & Mandel, T. Induction of leaf primordia by the cell wall protein expansin. Science 276, 1415–1418 (1997).
Peaucelle, A. et al. Arabidopsis phyllotaxis is controlled by the methyl-esterification status of cell-wall pectins. Curr. Biol. 18, 1943–1948 (2008).
Peaucelle, A. et al. Pectin-induced changes in cell wall mechanics underlie organ initiation in Arabidopsis. Curr. Biol. 21, 1720–1726 (2011).
Zhao, F. et al. Xyloglucans and microtubules synergistically maintain meristem geometry and phyllotaxis. Plant Phys. 181, 1191–1206 (2019).
Milani, P. et al. In vivo analysis of local wall stiffness at the shoot apical meristem in Arabidopsis using atomic force microscopy. Plant J. 67, 1116–1123 (2011).
Kierzkowski, D. et al. Elastic domains regulate growth and organogenesis in the plant shoot apical meristem. Science 335, 1096–1099 (2012).
Hamant, O., Inoue, D., Bouchez, D., Dumais, J. & Mjolsness, E. Are microtubules tension sensors? Nat. Commun. 10, 2360 (2019).
Uyttewaal, M. et al. Mechanical stress acts via Katanin to amplify differences in growth rate between adjacent cells in Arabidopsis. Cell 149, 439–451 (2012).
Heisler, M. G. et al. Alignment between PIN1 polarity and microtubule orientation in the shoot apical meristem reveals a tight coupling between morphogenesis and auxin transport. PLoS Biol. 8, e1000516 (2010).
Braybrook, S. A. & Peaucelle, A. Mechano-chemical aspects of organ formation in Arabidopsis thaliana: the relationship between auxin and pectin. PLoS ONE 8, e57813 (2013).
Nakayama, N. et al. Mechanical regulation of auxin-mediated growth. Curr. Biol. 22, 1468–1476 (2012).
Landrein, B. et al. Mechanical stress contributes to the expression of the STM homeobox gene in Arabidopsis shoot meristems. eLife 4, e07811 (2015).
Mirabet, V., Besnard, F., Vernoux, T. & Boudaoud, A. Noise and robustness in phyllotaxis. PLoS Comput. Biol. 8, e1002389 (2012).
Zhou, L. et al. Epidermal restriction confers robustness to organ shapes. J. Integr. Plant Biol. 62, 1853–1867 (2020).
Rasmussen, C. G. & Bellinger, M. An overview of plant division-plane orientation. New Phytol. 219, 505–512 (2018).
Louveaux, M., Julien, J.-D., Mirabet, V., Boudaoud, A. & Hamant, O. Cell division plane orientation based on tensile stress in Arabidopsis thaliana. Proc. Natl Acad. Sci. USA 113, E4294–E4303 (2016).
Sahlin, P. & Jönsson, H. A modeling study on how cell division affects properties of epithelial tissues under isotropic growth. PLoS ONE 5, e11750 (2010).
Jones, A. R. et al. Cell-size dependent progression of the cell cycle creates homeostasis and flexibility of plant cell size. Nat. Commun. 8, 15060 (2017).
Willis, L. et al. Cell size and growth regulation in the Arabidopsis thaliana apical stem cell niche. Proc. Natl Acad. Sci. USA 113, E8238–E8246 (2016).
Zhao, F. et al. Microtubule-mediated wall anisotropy contributes to leaf blade flattening. Curr. Biol. 30, 3972–3985 (2020).
Wyrzykowska, J., Pien, S., Shen, W.-H. & Fleming, A. J. Manipulation of leaf shape by modulation of cell division. Development 129, 957–964 (2002).
Leiboff, S. et al. Genetic control of morphometric diversity in the maize shoot apical meristem. Nat. Commun. 6, 8974 (2015).
Jackson, M. D. B. et al. Global topological order emerges through local mechanical control of cell divisions in the Arabidopsis shoot apical meristem. Cell Syst. 8, 53–65 (2019).
Long, Y. et al. Cellular heterogeneity in pressure and growth emerges from tissue topology and geometry. Curr. Biol. 30, 1504–1516 (2020).
Cheddadi, I., Génard, M., Bertin, N. & Godin, C. Coupling water fluxes with cell wall mechanics in a multicellular model of plant development. PLoS Comput. Biol. 15, e1007121 (2019).
Gisel, A., Barella, S., Hempel, F. D. & Zambryski, P. C. Temporal and spatial regulation of symplastic trafficking during development in Arabidopsis thaliana apices. Development 126, 1879–1889 (1999).
Rinne, P. L. & van der Schoot, C. Symplasmic fields in the tunica of the shoot apical meristem coordinate morphogenetic events. Development 125, 1477–1485 (1998).
Oparka, K. J. & Prior, D. A. M. Direct evidence for pressure-generated closure of plasmodesmata. Plant J. 2, 741–750 (1992).
Schulz, A. Plasmodesmal widening accompanies the short-term increase in symplasmic phloem unloading in pea root tips under osmotic stress. Protoplasma 188, 22–37 (1995).
Park, K., Knoblauch, J., Oparka, K. & Jensen, K. H. Controlling intercellular flow through mechanosensitive plasmodesmata nanopores. Nat. Commun. 10, 3564 (2019).
Hernández-Hernández, V., Benítez, M. & Boudaoud, A. Interplay between turgor pressure and plasmodesmata during plant development. J. Exp. Bot. 3, 763–10 (2019).
Haas, K. T., Wightman, R., Meyerowitz, E. M. & Peaucelle, A. Pectin homogalacturonan nanofilament expansion drives morphogenesis in plant epidermal cells. Science 367, 1003–1007 (2020).
Cosgrove, D. J. & Anderson, C. T. Plant cell growth: do pectins drive lobe formation in Arabidopsis pavement cells? Curr. Biol. 30, R660–R662 (2020).
Alber, M., Godin, C., Maini, P. K., Merks, R. & Mjolsness, E. Introduction. Bull. Math. Biol. 81, 3214–3218 (2019).
Prusinkiewicz, P. & Lindenmayer, A. The Algorithmic Beauty of Plants (Springer Verlag, 1990).
Boudon, F., Pradal, C., Cokelaer, T., Prusinkiewicz, P. & Godin, C. L-py: an L-system simulation framework for modeling plant architecture development based on a dynamic language. Front. Plant Sci. 3, 76 (2012).
Hemmerling, R., Kniemeyer, O., Lanwert, D., Kurth, W. & Buck-Sorlin, G. The rule-based language XL and the modelling environment GroIMP illustrated with simulated tree competition. Funct. Plant Biol. 35, 739–750 (2008).
Prusinkiewicz, P., Cieslak, M., Ferraro, P. & Hanan, J. Modeling plant development with L-systems. Math. Model. Plant Biol. 144, 139–169 (2018). Springer.
Walia, A., Waadt, R. & Jones, A. M. Genetically encoded biosensors in plants: pathways to discovery. Annu. Rev. Plant Biol. 69, 497–524 (2018).
Roca-Cusachs, P., Conte, V. & Trepat, X. Quantifying forces in cell biology. Nature 19, 742–751 (2017).
Acknowledgements
We thank G. Ingram, O. Hamant, Y. Jaillais and Y. Coudert for comments on the manuscript; our colleagues at the Laboratoire Reproduction et Développement des Plantes for fruitful discussions; and C. S. Galvan-Ampudia for providing the confocal microscope images for the figures. This work was supported by the European Union’s Horizon 2020 Robotic for Microfarms project (ROMI; grant agreement no. 773875) to T.V., F.B and C.G. and ANR-18-CE12-0014-02 (ChromAuxi) to T.V.
Author information
Authors and Affiliations
Contributions
T.V, F.B and C.G designed the figures and wrote the manuscript. F.B. constructed the figures.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Plants thanks Alexis Peaucelle, Yuling Jiao and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vernoux, T., Besnard, F. & Godin, C. What shoots can teach about theories of plant form. Nat. Plants 7, 716–724 (2021). https://doi.org/10.1038/s41477-021-00930-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-021-00930-0
This article is cited by
-
Evolution of meristem zonation by CLE gene duplication in land plants
Nature Plants (2022)