Abstract
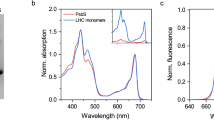
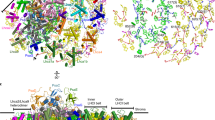
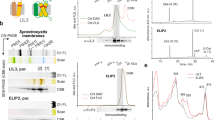
Photosystem I (PSI) is a major player in the light reactions of photosynthesis. In higher plants, it consists of a core complex and four external antennae, Lhca1–4 forming the PSI–light-harvesting complex I (LHCI) supercomplex. The protein and pigment composition as well as the spectroscopic properties of this complex are considered to be identical in different higher plant species. In addition to the four Lhca, a pool of mobile LHCII increases the antenna size of PSI under most light conditions. In this work, we have first investigated purified PSI complexes and then PSI in vivo upon long-term dark-adaptation of four well-studied plant species: Arabidopsis thaliana, Zea mays, Nicotiana tabacum and Hordeum vulgare. By performing time-resolved fluorescence measurements, we show that LHCII is associated with PSI also in a dark-adapted state in all the plant species investigated. The number of LHCII subunits per PSI is plant-dependent, varying between one and three. Furthermore, we show that the spectroscopic properties of PSI–LHCI supercomplexes differ in different plants.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The raw data that support the findings of this study are available from the corresponding author upon reasonable request. Source data are provided with this paper.
References
Jansson, S. A guide to the Lhc genes and their relatives in Arabidopsis. Trends Plant Sci. 4, 236–240 (1999).
Croce, R. & van Amerongen, H. Light-harvesting in photosystem I. Photosynth. Res. 116, 153–166 (2013).
Mazor, Y., Borovikova, A. & Nelson, N. The structure of plant photosystem I super-complex at 2.8 Å resolution. eLife 4, e07433 (2015).
Qin, X., Suga, M., Kuang, T. & Shen, J. R. Photosynthesis. Structural basis for energy transfer pathways in the plant PSI–LHCI supercomplex. Science 348, 989–995 (2015).
Allen, J. F. Botany. State transitions—a question of balance. Science 299, 1530–1532 (2003).
Allen, J. F. Plastoquinone redox control of chloroplast thylakoid protein phosphorylation and distribution of excitation energy between photosystems: discovery, background, implications. Photosynth. Res. 73, 139–148 (2002).
Osmond, B., Chow, W. S., Pogson, B. J. & Robinson, S. A. Probing functional and optical cross-sections of PSII in leaves during state transitions using fast repetition rate light induced fluorescence transients. Funct. Plant Biol. 46, 567–583 (2019).
Wood, W. H. J., Barnett, S. F. H., Flannery, S., Hunter, C. N. & Johnson, M. P. Dynamic thylakoid stacking is regulated by LHCII phosphorylation but not its interaction with PSI. Plant Physiol. 180, 2152–2166 (2019).
Ancín, M. et al. Overexpression of thioredoxin m in tobacco chloroplasts inhibits the protein kinase STN7 and alters photosynthetic performance. J. Exp. Bot. 70, 1005–1016 (2019).
Mekala, N. R., Suorsa, M., Rantala, M., Aro, E.-M. & Tikkanen, M. Plants actively avoid state transitions upon changes in light intensity: role of light-harvesting complex II protein dephosphorylation in high light. Plant Physiol. 168, 721–734 (2015).
Wientjes, E., van Amerongen, H. & Croce, R. LHCII is an antenna of both photosystems after long-term acclimation. Biochim. Biophys. Acta Bioenerg. 1827, 420–426 (2013).
Allen, J. F. Why we need to know the structure of phosphorylated chloroplast light-harvesting complex II. Physiol. Plant. 161, 28–44 (2017).
Bos, P. et al. Digitonin-sensitive LHCII enlarges the antenna of photosystem I in stroma lamellae of Arabidopsis thaliana after far-red and blue-light treatment. Biochim. Biophys. Acta Bioenerg. 1860, 651–658 (2019).
Galka, P. et al. Functional analyses of the plant photosystem I–light-harvesting complex II supercomplex reveal that light-harvesting complex II loosely bound to photosystem II Is a very efficient antenna for photosystem I in state II. Plant Cell 24, 2963–2978 (2012).
Kouřil, R. et al. Structural characterization of a complex of photosystem I and light-harvesting complex II of Arabidopsis thaliana. Biochemistry 44, 10935–10940 (2005).
Pan, X. et al. Structure of the maize photosystem I supercomplex with light-harvesting complexes I and II. Science 360, 1109–1113 (2018).
Bell, A. J., Frankel, L. K. & Bricker, T. M. High yield non-detergent isolation of photosystem I–light-harvesting chlorophyll II membranes from spinach thylakoids: implications for the organization of the PS I antennae in higher plants. J. Biol. Chem. 290, 18429–18437 (2015).
Bos, I. et al. Multiple LHCII antennae can transfer energy efficiently to a single photosystem I. Biochim. Biophys. Acta Bioenerg. 1858, 371–378 (2017).
Yadav, K. N. et al. Supercomplexes of plant photosystem I with cytochrome b6f, light-harvesting complex II and NDH. Biochim. Biophys. Acta Bioenerg. 1858, 12–20 (2017).
Benson, S. L. et al. An intact light harvesting complex I antenna system is required for complete state transitions in Arabidopsis. Nat. Plants 1, 15176 (2015).
Akhtar, P. et al. Excitation energy transfer between light-harvesting complex II and photosystem I in reconstituted membranes. Biochim. Biophys. Acta Bioenerg. 1857, 462–472 (2016).
Mazor, Y., Borovikova, A., Caspy, I. & Nelson, N. Structure of the plant photosystem I supercomplex at 2.6 Å resolution. Nat. Plants 3, 1–9 (2017).
Nelson, N. & Junge, W. Structure and energy transfer in photosystems of oxygenic photosynthesis. Annu. Rev. Biochem. 84, 659–683 (2015).
Wientjes, E., van Stokkum, I. H., van Amerongen, H. & Croce, R. The role of the individual Lhcas in photosystem I excitation energy trapping. Biophys. J. 101, 745–754 (2011).
Croce, R., Dorra, D., Holzwarth, A. R. & Jennings, R. C. Fluorescence decay and spectral evolution in intact photosystem I of higher plants. Biochemistry 39, 6341–6348 (2000).
Santabarbara, S., Tibiletti, T., Remelli, W. & Caffarri, S. Kinetics and heterogeneity of energy transfer from light harvesting complex II to photosystem I in the supercomplex isolated from Arabidopsis. Phys. Chem. Chem. Phys. 19, 9210–9222 (2017).
Ihalainen, J. A. et al. Excitation energy trapping in photosystem I complexes depleted in Lhca1 and Lhca4. FEBS Lett. 579, 4787–4791 (2005).
Morosinotto, T., Breton, J., Bassi, R. & Croce, R. The nature of a chlorophyll ligand in Lhca proteins determines the far red fluorescence emission typical of photosystem I. J. Biol. Chem. 278, 49223–49229 (2003).
Jennings, R. C., Zucchelli, G. & Santabarbara, S. Photochemical trapping heterogeneity as a function of wavelength, in plant photosystem I (PSI–LHCI). Biochim. Biophys. Acta Bioenerg. 1827, 779–785 (2013).
van Oort, B. et al. Picosecond fluorescence of intact and dissolved PSI–LHCI crystals. Biophys. J. 95, 5851–5861 (2008).
Le Quiniou, C. et al. PSI–LHCI of Chlamydomonas reinhardtii: increasing the absorption cross section without losing efficiency. Biochim. Biophys. Acta Bioenerg. 1847, 458–467 (2015).
van Oort, B. et al. Effect of antenna-depletion in photosystem II on excitation energy transfer in Arabidopsis thaliana. Biophys. J. 98, 922–931 (2010).
Chukhutsina, V. U., Büchel, C. & van Amerongen, H. Variations in the first steps of photosynthesis for the diatom Cyclotella meneghiniana grown under different light conditions. Biochim. Biophys. Acta Bioenerg. 1827, 10–18 (2013).
Wientjes, E., van Amerongen, H. & Croce, R. Quantum yield of charge separation in photosystem II: functional effect of changes in the antenna size upon light acclimation. J. Phys. Chem. B 117, 11200–11208 (2013).
Chukhutsina, V., Bersanini, L., Aro, E.-M. & van Amerongen, H. Cyanobacterial flv4-2 operon-encoded proteins optimize light harvesting and charge separation in photosystem II. Mol. Plant 8, 747–761 (2015).
Tian, L., Xu, P., Chukhutsina, V. U., Holzwarth, A. R. & Croce, R. Zeaxanthin-dependent nonphotochemical quenching does not occur in photosystem I in the higher plant Arabidopsis thaliana. Proc. Natl Acad. Sci. USA 114, 4828–4832 (2017).
Wientjes, E. & Croce, R. PMS: photosystem I electron donor or fluorescence quencher. Photosynth. Res. 111, 185–191 (2012).
Miloslavina, Y. et al. Charge separation kinetics in intact photosystem II core particles is trap-limited. A picosecond fluorescence study. Biochemistry 45, 2436–2442 (2006).
Szczepaniak, M. et al. Charge separation, stabilization, and protein relaxation in photosystem II core particles with closed reaction center. Biophys. J. 96, 621–631 (2009).
Tian, L., Farooq, S. & van Amerongen, H. Probing the picosecond kinetics of the photosystem II core complex in vivo. Phys. Chem. Chem. Phys. 15, 3146–3154 (2013).
Wientjes, E., van Stokkum, I. H., van Amerongen, H. & Croce, R. Excitation-energy transfer dynamics of higher plant photosystem I light-harvesting complexes. Biophys. J. 100, 1372–1380 (2011).
Schatz, G. H., Brock, H. & Holzwarth, A. R. Kinetic and energetic model for the primary processes in photosystem II. Biophys. J. 54, 397–405 (1988).
Tian, L., van Stokkum, I. H., Koehorst, R. B. & van Amerongen, H. Light harvesting and blue-green light induced non-photochemical quenching in two different C-phycocyanin mutants of Synechocystis PCC 6803. J. Phys. Chem. B 117, 11000–11006 (2013).
Pietrzykowska, M. et al. The light-harvesting chlorophyll a/b binding proteins Lhcb1 and Lhcb2 play complementary roles during state transitions in Arabidopsis. Plant Cell 26, 3646–3660 (2014).
Amerongen, H. van, Grondelle, R. van & Valkunas, L. Photosynthetic Excitons (World Scientific, 2010).
Barter, L. M. C. et al. Relationship between excitation energy transfer, trapping, and antenna size in photosystem II. Biochemistry 40, 4026–4034 (2001).
Wientjes, E., Oostergetel, G. T., Jansson, S., Boekema, E. J. & Croce, R. The role of Lhca complexes in the supramolecular organization of higher plant photosystem I. J. Biol. Chem. 284, 7803–7810 (2009).
Morosinotto, T., Castelletti, S., Breton, J., Bassi, R. & Croce, R. Mutation analysis of Lhca1 antenna complex: low energy absorption forms originate from pigment–pigment interactions. J. Biol. Chem. 77, 36253–36261 (2002).
Mozzo, M., Morosinotto, T., Bassi, R. & Croce, R. Probing the structure of Lhca3 by mutation analysis. Biochim. Biophys. Acta Bioenerg. 1757, 1607–1613 (2006).
Croce, R. et al. Origin of the 701-nm fluorescence emission of the Lhca2 subunit of higher plant photosystem I. J. Biol. Chem. 279, 48543–48549 (2004).
Morosinotto, T., Mozzo, M., Bassi, R. & Croce, R. Pigment–pigment interactions in Lhca4 antenna complex of higher plants photosystem I. J. Biol. Chem. 280, 20612–20619 (2005).
Suorsa, M. et al. Light acclimation involves dynamic re-organization of the pigment–protein megacomplexes in non-appressed thylakoid domains. Plant J. 84, 360–373 (2015).
Wientjes, E., Drop, B., Kouril, R., Boekema, E. J. & Croce, R. During state 1 to state 2 transition in Arabidopsis thaliana, the photosystem II supercomplex gets phosphorylated but does not disassemble. J. Biol. Chem. 288, 32821–32826 (2013).
Pribil, M., Pesaresi, P., Hertle, A., Barbato, R. & Leister, D. Role of plastid protein phosphatase TAP38 in LHCII dephosphorylation and thylakoid electron flow. PLoS Biol. 8, e1000288 (2010).
Haldrup, A., Jensen, P. E., Lunde, C. & Scheller, H. V. Balance of power: a view of the mechanism of photosynthetic state transitions. Trends Plant Sci. 6, 301–305 (2001).
Crepin, A. & Caffarri, S. The specific localizations of phosphorylated Lhcb1 and Lhcb2 isoforms reveal the role of Lhcb2 in the formation of the PSI–LHCII supercomplex in Arabidopsis during state transitions. Biochim. Biophys. Acta Bioenerg. 1847, 1539–1548 (2015).
Longoni, P., Douchi, D., Cariti, F., Fucile, G. & Goldschmidt-Clermont, M. Phosphorylation of the light-harvesting complex II isoform Lhcb2 Is central to state transitions. Plant Physiol. 169, 2874–2883 (2015).
Xu, P., Tian, L., Kloz, M. & Croce, R. Molecular insights into Zeaxanthin-dependent quenching in higher plants. Sci. Rep. 5, 13679 (2015).
Dinc, E., Ramundo, S., Croce, R. & Rochaix, J. D. Repressible chloroplast gene expression in Chlamydomonas: a new tool for the study of the photosynthetic apparatus. Biochim. Biophys. Acta Bioenerg. 1837, 1548–1552 (2014).
Nellaepalli, S., Kodru, S., Tirupathi, M. & Subramanyam, R. Anaerobiosis induced state transition: a non photochemical reduction of PQ pool mediated by NDH in Arabidopsis thaliana. PLoS ONE 7, e49839 (2012).
Croce, R., Zucchelli, G., Garlaschi, F. M. & Jennings, R. C. A thermal broadening study of the antenna chlorophylls in PSI-200, LHCI, and PSI core. Biochemistry 37, 17355–17360 (1998).
Porra, R. J., Thompson, W. A. & Kriedemann, P. E. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim. Biophys. Acta Bioenerg. 975, 384–394 (1989).
Edgar, R. C. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32, 1792–1797 (2004).
Chukhutsina, V. U., Holzwarth, A. R. & Croce, R. Time-resolved fluorescence measurements on leaves: principles and recent developments. Photosynth. Res. 140, 355–369 (2018).
Digris, A. V. et al. Thermal stability of a flavoprotein assessed from associative analysis of polarized time-resolved fluorescence spectroscopy. Eur. Biophys. J. 28, 526–531 (1999).
van Stokkum, I. H. M., Larsen, D. S. & van Grondelle, R. Global and target analysis of time-resolved spectra. Biochim. Biophys. Acta Bioenerg. 1657, 82–104 (2004).
Wientjes, E. & Croce, R. The light-harvesting complexes of higher-plant photosystem I: Lhca1/4 and Lhca2/3 form two red-emitting heterodimers. Biochem. J. 433, 477–485 (2011).
Acknowledgements
This work was supported by the Dutch Organization for scientific research (NWO) via Vici grant no. 86510013 to R.C. X.L was supported by the China Scholarship Council. We thank R. Fristedt for help with growing the plants.
Author information
Authors and Affiliations
Contributions
R.C. and V.C. conceived the project, designed the study and wrote the manuscript. V.C. performed most of the experiments and data analysis. P.X. and X.L. performed biochemical experiments. All authors revised the manuscript and approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Plants thanks John Allen, Matthew Johnson and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary discussion, Figs. 1–6 and Tables 1 and 2.
Source data
Source Data Fig. 1
Unprocessed SDS–PAGE.
Source Data Supplementary Fig. 4
Unprocessed chemiluminescence signals and Ponceau staining.
Rights and permissions
About this article
Cite this article
Chukhutsina, V.U., Liu, X., Xu, P. et al. Light-harvesting complex II is an antenna of photosystem I in dark-adapted plants. Nat. Plants 6, 860–868 (2020). https://doi.org/10.1038/s41477-020-0693-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-020-0693-4
This article is cited by
-
Exogenous melatonin enhanced cadmium stress tolerance of cucumber seedlings (Cucumis sativus L.)
Biologia (2024)
-
Light-induced changes of far-red excited chlorophyll fluorescence: further evidence for variable fluorescence of photosystem I in vivo
Photosynthesis Research (2023)
-
Spectral diversity of photosystem I from flowering plants
Photosynthesis Research (2023)
-
A perspective on the major light-harvesting complex dynamics under the effect of pH, salts, and the photoprotective PsbS protein
Photosynthesis Research (2023)
-
Spruce versus Arabidopsis: different strategies of photosynthetic acclimation to light intensity change
Photosynthesis Research (2022)