Abstract
Although hundreds of plant lineages have independently evolved dioecy (that is, separation of the sexes), the underlying genetic basis remains largely elusive1. Here we show that diverse poplar species carry partial duplicates of the ARABIDOPSIS RESPONSE REGULATOR 17 (ARR17) orthologue in the male-specific region of the Y chromosome. These duplicates give rise to small RNAs apparently causing male-specific DNA methylation and silencing of the ARR17 gene. CRISPR–Cas9-induced mutations demonstrate that ARR17 functions as a sex switch, triggering female development when on and male development when off. Despite repeated turnover events, including a transition from the XY system to a ZW system, the sex-specific regulation of ARR17 is conserved across the poplar genus and probably beyond. Our data reveal how a single-gene-based mechanism of dioecy can enable highly dynamic sex-linked regions and contribute to maintaining recombination and integrity of sex chromosomes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The DNA- and RNA-seq data have been deposited in NCBI’s SRA under the accession number PRJNA542603. The genome assemblies have been deposited at ftp://plantgenie.org/Publications/Muller2019/.
References
Henry, I. M., Akagi, T., Tao, R. & Comai, L. One hundred ways to invent the sexes: theoretical and observed paths to dioecy in plants. Annu. Rev. Plant Biol. 69, 553–575 (2018).
McDonald, M. J., Rice, D. P. & Desai, M. M. Sex speeds adaptation by altering the dynamics of molecular evolution. Nature 531, 233–236 (2016).
Bachtrog, D. et al. Sex determination: why so many ways of doing it? PLoS Biol. 12, e1001899 (2014).
Renner, S. S. The relative and absolute frequencies of angiosperm sexual systems: dioecy, monoecy, gynodioecy, and an updated online database. Am. J. Bot. 101, 1588–1596 (2014).
Charlesworth, B. & Charlesworth, D. A model for the evolution of dioecy and gynodioecy. Am. Nat. 112, 975–997 (1978).
Akagi, T. et al. Two Y-chromosome-encoded genes determine sex in kiwifruit. Nat. Plants 5, 801–809 (2019).
Harkess, A. et al. Sex determination by two Y-linked genes in garden asparagus. Plant Cell https://doi.org/10.1105/tpc.19.00859(2020).
Renner, S. S. Pathways for making unisexual flowers and unisexual plants: moving beyond the ‘two mutations linked on one chromosome’ model. Am. J. Bot. 103, 587–589 (2016).
Akagi, T., Henry, I. M., Tao, R. & Comai, L. A Y-chromosome-encoded small RNA acts as a sex determinant in persimmons. Science 346, 646–650 (2014).
West, N. W. & Golenberg, E. M. Gender-specific expression of GIBBERELLIC ACID INSENSITIVE is critical for unisexual organ initiation in dioecious Spinacia oleracea. New Phytol. 217, 1322–1334 (2018).
Yang, H. W., Akagi, T., Kawakatsu, T. & Tao, R. Gene networks orchestrated by MeGI: a single-factor mechanism underlying sex determination in persimmon. Plant J. 98, 97–111 (2019).
Kersten, B., Pakull, B., Groppe, K., Lueneburg, J. & Fladung, M. The sex-linked region in Populus tremuloides Turesson 141 corresponds to a pericentromeric region of about two million base pairs on P. trichocarpa chromosome 19. Plant Biol. (Stuttg.) 16, 411–418 (2014).
Geraldes, A. et al. Recent Y chromosome divergence despite ancient origin of dioecy in poplars (Populus). Mol. Ecol. 24, 3243–3256 (2015).
McKown, A. D. et al. Sexual homomorphism in dioecious trees: extensive tests fail to detect sexual dimorphism in Populus. Sci. Rep. 7, 1831 (2017).
Tuskan, G. A. et al. The genome of black cottonwood, Populus trichocarpa (Torr. & Gray). Science 313, 1596–1604 (2006).
Wang, J., Street, N. R., Scofield, D. G. & Ingvarsson, P. K. Variation in linked selection and recombination drive genomic divergence during allopatric speciation of European and American aspens. Mol. Biol. Evol. 33, 1754–1767 (2016).
Pakull, B., Kersten, B., Lüneburg, J. & Fladung, M. A simple PCR-based marker to determine sex in aspen. Plant Biol. (Stuttg.) 17, 256–261 (2015).
Bräutigam, K. et al. Sexual epigenetics: gender-specific methylation of a gene in the sex determining region of Populus balsamifera. Sci. Rep. 7, 45388 (2017).
Aufsatz, W., Mette, M. F., van der Winden, J., Matzke, A. J. & Matzke, M. RNA-directed DNA methylation in Arabidopsis. Proc. Natl Acad. Sci. USA 99(Suppl. 4), 16499–16506 (2002).
Zhang, H. et al. Precocious flowering in trees: the FLOWERING LOCUS T gene as a research and breeding tool in Populus. J. Exp. Bot. 61, 2549–2560 (2010).
Kazama, Y. et al. A new physical mapping approach refines the sex-determining gene positions on the Silene latifolia Y-chromosome. Sci. Rep. 6, 18917 (2016).
Paolucci, I. et al. Genetic linkage maps of Populus alba L. and comparative mapping analysis of sex determination across Populus species. Tree Genet. Genomes 6, 863–875 (2010).
Tennessen, J. A. et al. Repeated translocation of a gene cassette drives sex-chromosome turnover in strawberries. PLoS Biol. 16, e2006062 (2018).
Zhou, R. et al. A willow sex chromosome reveals convergent evolution of complex palindromic repeats. Genome Biol. 21, 38 (2020).
Xiao, H., Jiang, N., Schaffner, E., Stockinger, E. J. & van der Knaap, E. A retrotransposon-mediated gene duplication underlies morphological variation of tomato fruit. Science 319, 1527–1530 (2008).
Harkess, A. et al. The asparagus genome sheds light on the origin and evolution of a young Y chromosome. Nat. Commun. 8, 1279 (2017).
Akagi, T. et al. The persimmon genome reveals clues to the evolution of a lineage-specific sex determination system in plants. PLoS Genet. 16, e1008566 (2020).
Capel, B. Vertebrate sex determination: evolutionary plasticity of a fundamental switch. Nat. Rev. Genet. 18, 675–689 (2017).
Boualem, A. et al. A cucurbit androecy gene reveals how unisexual flowers develop and dioecy emerges. Science 350, 688–691 (2015).
Jones, D. F. Unisexual maize plants and their bearing on sex differentiation in other plants and in animals. Genetics 19, 552–567 (1934).
To, J. P. et al. Type-A Arabidopsis response regulators are partially redundant negative regulators of cytokinin signaling. Plant Cell 16, 658–671 (2004).
Golenberg, E. M. & West, N. W. Hormonal interactions and gene regulation can link monoecy and environmental plasticity to the evolution of dioecy in plants. Am. J. Bot. 100, 1022–1037 (2013).
Almeida, P. et al. Single-molecule genome assembly of the basket willow, Salix viminalis, reveals earliest stages of sex chromosome expansion. Preprint at https://www.biorxiv.org/content/10.1101/589804v1 (2019).
Lin, Y. C. et al. Functional and evolutionary genomic inferences in Populus through genome and population sequencing of American and European aspen. Proc. Natl Acad. Sci. USA 115, E10970–E10978 (2018).
Charlesworth, D. Plant sex chromosomes. Annu. Rev. Plant Biol. 67, 397–420 (2016).
Robinson, K. M. et al. Populus tremula (European aspen) shows no evidence of sexual dimorphism. BMC Plant Biol. 14, 276 (2014).
Pucholt, P., Wright, A. E., Liu Conze, L., Mank, J. E. & Berlin, S. Recent sex chromosome divergence despite ancient dioecy in the willow Salix viminalis. Mol. Biol. Evol. 34, 1991–2001 (2017).
Okazaki, Y., Takahata, S., Hirakawa, H., Suzuki, Y. & Onodera, Y. Molecular evidence for recent divergence of X- and Y-linked gene pairs in Spinacia oleracea L. PLoS ONE 14, e0214949 (2019).
Veltsos, P. et al. Early sex-chromosome evolution in the diploid dioecious plant Mercurialis annua. Genetics 212, 815–835 (2019).
Sousa, A., Fuchs, J. & Renner, S. S. Cytogenetic comparison of heteromorphic and homomorphic sex chromosomes in Coccinia (Cucurbitaceae) points to sex chromosome turnover. Chromosome Res. 25, 191–200 (2017).
Hoenicka, H., Lehnhardt, D., Briones, V., Nilsson, O. & Fladung, M. Low temperatures are required to induce the development of fertile flowers in transgenic male and female early flowering poplar (Populus tremula L.). Tree Physiol. 36, 667–677 (2016).
Widholm, J. M. The use of fluorescein diacetate and phenosafranine for determining viability of cultured plant cells. Stain Technol. 47, 189–194 (1972).
Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120 (2014).
Schiffthaler, B. et al. An improved genome assembly of the European aspen Populus tremula. Preprint at https://www.biorxiv.org/content/10.1101/805614v1 (2019).
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012).
Luquez, V. et al. Natural phenological variation in aspen (Populus tremula): the SwAsp collection. Tree Genet. Genomes 4, 279–292 (2008).
Wang, J. et al. A major locus controls local adaptation and adaptive life history variation in a perennial plant. Genome Biol. 19, 72 (2018).
Zhou, X. & Stephens, M. Genome-wide efficient mixed-model analysis for association studies. Nat. Genet. 44, 821–824 (2012).
Storey, J. D., Bass, A. J., Dabney, A. & Robinson, D. qvalue: q-value estimation for false discovery rate control. R package version 2.18.0 http://github.com/jdstorey/qvalue (2019).
Inglis, P. W., Pappas, M. C. R., Resende, L. V. & Grattapaglia, D. Fast and inexpensive protocols for consistent extraction of high quality DNA and RNA from challenging plant and fungal samples for high-throughput SNP genotyping and sequencing applications. PLoS ONE 13, e0206085 (2018).
Schalamun, M. et al. Harnessing the MinION: an example of how to establish long-read sequencing in a laboratory using challenging plant tissue from Eucalyptus pauciflora. Mol. Ecol. Resour. 19, 77–89 (2019).
Dumolin, S., Demesure, B. & Petit, R. J. Inheritance of chloroplast and mitochondrial genomes in pedunculate oak investigated with an efficient PCR method. Theor. Appl. Genet. 91, 1253–1256 (1995).
Wick, R. R., Judd, L. M. & Holt, K. E. Performance of neural network basecalling tools for Oxford Nanopore sequencing. Genome Biol. 20, 129 (2019).
Koren, S. et al. Canu: scalable and accurate long-read assembly via adaptive k-mer weighting and repeat separation. Genome Res. 27, 722–736 (2017).
Walker, B. J. et al. Pilon: an integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS ONE 9, e112963 (2014).
Roach, M. J., Schmidt, S. A. & Borneman, A. R. Purge Haplotigs: allelic contig reassignment for third-gen diploid genome assemblies. BMC Bioinform. 19, 460 (2018).
Simao, F. A., Waterhouse, R. M., Ioannidis, P., Kriventseva, E. V. & Zdobnov, E. M. BUSCO: assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics 31, 3210–3212 (2015).
Stanke, M., Diekhans, M., Baertsch, R. & Haussler, D. Using native and syntenically mapped cDNA alignments to improve de novo gene finding. Bioinformatics 24, 637–644 (2008).
Alonge, M. et al. RaGOO: fast and accurate reference-guided scaffolding of draft genomes. Genome Biol. 20, 224 (2019).
Kurtz, S. et al. Versatile and open software for comparing large genomes. Genome Biol. 5, R12 (2004).
Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 17, 10–12 (2011).
Andrews, S. FastQC: a quality control tool for high throughput sequence data http://www.bioinformatics.babraham.ac.uk/projects/fastqc (2010).
Krueger, F. & Andrews, S. R. Bismark: a flexible aligner and methylation caller for Bisulfite-Seq applications. Bioinformatics 27, 1571–1572 (2011).
Chang, S., Puryear, J. & Cairney, J. A simple and efficient method for isolating RNA from pine trees. Plant Mol. Biol. Rep. 11, 113–116 (1993).
Street, N. R. et al. The genetics and genomics of the drought response in Populus. Plant J. 48, 321–341 (2006).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Anders, S., Pyl, P.T. & Huber, W. HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169 (2015).
Lorenz, R. et al. ViennaRNA package 2.0. Algorithms Mol. Biol. 6, 26 (2011).
Kohany, O., Gentles, A. J., Hankus, L. & Jurka, J. Annotation, submission and screening of repetitive elements in Repbase: RepbaseSubmitter and Censor. BMC Bioinform. 7, 474 (2006).
Robinson, J. T. et al. Integrative Genomics Viewer. Nat. Biotechnol. 29, 24–26 (2011).
Schmittgen, T. D. & Livak, K. J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 3, 1101–1108 (2008).
Liu, H. et al. CRISPR-P 2.0: an improved CRISPR–Cas9 tool for genome editing in plants. Mol. Plant 10, 530–532 (2017).
Bruegmann, T., Polak, O., Deecke, K., Nietsch, J. & Fladung, M. Poplar transformation. Methods Mol. Biol. 1864, 165–177 (2019).
Fladung, M., Kaufmann, H., Markussen, T. & Hoenicka, H. Construction of a Populus tremuloides Michx. BAC library. Silvae Genetica 57, 65–69 (2008).
Poplin, R. et al. Scaling accurate genetic variant discovery to tens of thousands of samples. Preprint at https://www.biorxiv.org/content/10.1101/201178v3 (2018).
Knaus, B. J. & Grunwald, N. J. vcfR: a package to manipulate and visualize variant call format data in R. Mol. Ecol. Resour. 17, 44–53 (2017).
Ma, T. et al. Genomic insights into salt adaptation in a desert poplar. Nat. Commun. 4, 2797 (2013).
Yang, W. et al. The draft genome sequence of a desert tree Populus pruinosa. Gigascience 6, 1–7 (2017).
Tamura, K. & Nei, M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 10, 512–526 (1993).
Kumar, S., Stecher, G. & Tamura, K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 33, 1870–1874 (2016).
R: a language and environment for statistical computing R 3.6.1 (R Core Team, 2019).
Lawrence, M. et al. Software for computing and annotating genomic ranges. PLoS Comput. Biol. 9, e1003118 (2013).
Charif, D. & Lobry, J. in Structural Approaches to Sequence Evolution: Molecules, Networks, Populations (eds Bastolla, U. et al.) 207–232 (Springer Berlin Heidelberg, 2007).
Wickham, H. ggplot2: Elegant Graphics for Data Analysis 216 (Springer, 2009).
Acknowledgements
We thank members of the Thünen Institute of Forest Genetics and S. DiFazio, M. Olson and G. Tuskan for helpful comments and discussions; J. Mank and T. Brügmann for critical reading of the manuscript; S. Müller for language editing; and K. Groppe, A. Eikhof, D. Ebbinghaus, G. Wiemann, D. Boedecker, M. Wellern, A. Worm, M. Spauszus, L. Lierke and J. Lüneburg for technical assistance. We acknowledge funding from grants of the Deutsche Forschungsgemeinschaft to N.A.M. (DFG grant no. MU 4357/1-1) and M.F. (DFG grant no. FL 263/15-1). N.R.S., N.M., Z.C.L., V.K. and K.M.R. acknowledge funding from Trees for the Future (T4F), the Knut and Alice Wallenberg Foundation, the Umeå Plant Science Centre Berzelii Centre, the Stiftelsen för Strategisk Forskning Centre for Plant Developmental Biology, the Kempe Foundation and the Swedish Research Council Vetenskapsrådet. We thank the Swedish National Genomics Infrastructure hosted at SciLifeLab, the National Bioinformatics Infrastructure Sweden (NBIS), for providing computational assistance and the Uppsala Multidisciplinary Center for Advanced Computational Science for providing computational infrastructure. We further acknowledge funding from the Natural Sciences and Engineering Research Council of Canada (NSERC) to K.B. (grant no. RGPIN-2017-06552) and Q.C. (grant no. RGPIN-2019-04041).
Author information
Authors and Affiliations
Contributions
N.A.M., B.K., Q.C., N.R.S. and M.F. conceptualized the project. N.A.M., B.K., A.P.L.M., N.M., C.B., K.B., Z.C.L., M.M., B.P., K.M.R., N.R.S. and M.F. conceived the methodology. N.A.M., B.K., A.P.L.M., N.M., C.B., K.B., Z.C.L., H.H., V.K., B.P. and N.R.S. performed experiments and collected the data. N.A.M., B.K., A.P.L.M., N.M., C.B., K.B., M.M., K.M.R., P.K.I. and N.R.S. analysed the data. M.S. and C.V. provided resources. N.A.M., B.K., N.R.S. and M.F. acquired the funding. N.A.M., B.K., P.K.I., Q.C., N.R.S. and M.F. supervised the project. N.A.M. and N.R.S. validated the data. N.A.M., N.M., K.B., K.M.R. and P.K.I. conducted the visualization. N.A.M. wrote the paper, and all authors reviewed and edited it.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Plants thanks Roberta Bergero, Susanne Renner and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
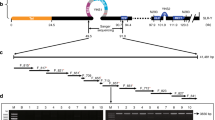
Extended Data Fig. 1 Male-specific long non-coding RNAs span TOZ19 and the ARR17 inverted repeat.
a, mRNA-seq data mapped against the new male P. tremula genome assembly (Methods) for seven female and five male biologically independent aspen samples. TOZ19 is represented by the left rightward pointing arrow, the ARR17 inverted repeat by the two small arrows pointing towards each other. Coverage on the forward strand is indicated by lines above zero and coverage on the reverse strand by lines below zero. b, Integrative Genomics Viewer (IGV)70 screenshot of the same RNA-seq data shown in (a). In males, transcription starts from the TOZ19 transcriptional start site. The ARR17 inverted repeat mainly behaves as an intron. The few reads mapping directly to the inverted repeat may represent alternative transcript isoforms. No reads map to the ARR17 gene (data not shown). c, Repeats identified by the CENSOR webtool69 with an alignment score > 2,000. The right part of the ‘TOZ19/ARR17 inverted repeat transcript’ is generated from a sequence with partial identity to an En-Spm3 DNA transposon.
Extended Data Fig. 2 Size distribution of the small RNAs mapping to the ARR17 inverted repeat.
Summed number of alignments of female (n = 11 biologically independent samples) and male (n = 7 biologically independent samples) aspen small RNAs mapping to the ARR17 inverted repeat region of the new male P. tremula genome assembly (Methods). Most reads map to 1-5 mapping locations, which corresponds to the total number of ARR17 sequences: the ARR17 gene, the two arms of the ARR17 inverted repeat and two additional male-specific partial ARR17 fragments. All multi-mapping reads > 18 bp mapped exclusively to these five ARR17 sequence features. Data are from two independent experiments.
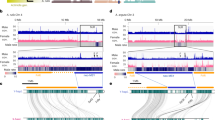
Extended Data Fig. 3 The ARR17 locus exhibits male-specific methylation coinciding with the Y-chromosomal duplicated part.
a, Mean percent methylation along the ARR17 genomic region of the P. trichocarpa genome based on bisulfite-sequencing of 9 female (magenta) and 12 male (cyan) biologically independent P. balsamifera samples18. The location of ARR17 is indicated by an arrow. Colored shading marks the standard error. Intron 4, which represents a Copia/LTR sequence (Supplementary Note 2), is highly methylated in both sexes. The first half of ARR17 is only methylated in males. b, Percent methylation along the ARR17 genomic region of the P. tremula genome assembly based on bisulfite-sequencing of two female (Asp201 and Asp044) and three male (Asp005, Asp113 and Asp116) biologically independent P. tremula samples. The location of ARR17 is indicated by an arrow. Again the first half of ARR17 is only methylated in males.
Extended Data Fig. 4 P. tremula flower buds exhibit female-specific ARR17 expression.
Relative ARR17 expression as determined by qRT-PCR for n = 3 biologically independent flower buds/young catkins of each of eight field-grown P. tremula genotypes (females: Brauna11, Tio13-67, W7 and W97; males: Bliz153, C44, Rend14-67 and W52). UBQ (Potri.001G418500) was used as a reference gene for normalization. Boxplots show upper and lower quartiles (box limits), the median (center line) and the 1.5* interquartile range (whiskers).
Extended Data Fig. 5 Masculinized arr17 flowers develop viable pollen.
a, Dehiscing anthers of a representative flower of an early-flowering arr17 mutant line release white pollen grains. Photo shows 40⨯ magnification. The experiment was repeated twice with similar results. b, Fluorescein diacetate (FDA) staining indicates pollen viability42. Photo shows 200⨯ magnification of stained pollen grains.
Extended Data Fig. 6 Pool-sequencing reveals a single region of chromosome 19 exhibiting marked female-specific coverage in P. alba.
a, The number of 1 kbp regions with sex-specific coverage (exceeding at least 50% of the expected haploid coverage), indicative of hemizygosity, for a pool of seven female (magenta) and seven male (cyan) white poplar (P. alba) individuals in sliding windows (window = 100 kbp, step=25 kbp). DNA-seq data of the pools were mapped against the chromosome-level P. tremula v2.2 genome assembly44. b, DNA-seq coverage for the two P. alba pools, zooming into the major hemizygous region on chromosome 19. The location of the ARR17 gene is indicated by a gray arrow.
Supplementary information
Supplementary Information
Supplementary Figs. 1–7 and Notes 1 and 2.
Supplementary Tables
Supplementary Tables 1–8.
Supplementary Data 2
Multifasta file of all ARR17 and ARR17 inverted repeat sequences used for the phylogenetic analysis shown in Fig. 4.
Rights and permissions
About this article
Cite this article
Müller, N.A., Kersten, B., Leite Montalvão, A.P. et al. A single gene underlies the dynamic evolution of poplar sex determination. Nat. Plants 6, 630–637 (2020). https://doi.org/10.1038/s41477-020-0672-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-020-0672-9
This article is cited by
-
Genetic insights into the dissolution of dioecy in diploid persimmon Diospyros oleifera Cheng
BMC Plant Biology (2023)
-
Genomic profiling of dioecious Amaranthus species provides novel insights into species relatedness and sex genes
BMC Biology (2023)
-
Comparative transcriptomic analysis of male and females in the dioecious weeds Amaranthus palmeri and Amaranthus tuberculatus
BMC Plant Biology (2023)
-
Recurrent neo-sex chromosome evolution in kiwifruit
Nature Plants (2023)
-
Repetitive DNA sequence detection and its role in the human genome
Communications Biology (2023)