Abstract
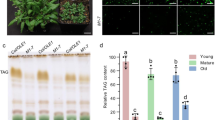
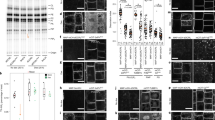
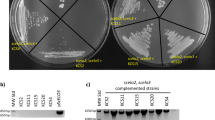
Plants strictly regulate the levels of sterol in their cells, as high sterol levels are toxic. However, how plants achieve sterol homeostasis is not fully understood. We isolated an Arabidopsis thaliana mutant that abundantly accumulated sterol esters in structures of about 1 µm in diameter in leaf cells. We designated the mutant high sterol ester 1 (hise1) and called the structures sterol ester bodies. Here, we show that HISE1, the gene product that is altered in this mutant, functions as a key factor in plant sterol homeostasis on the endoplasmic reticulum (ER) and participates in a fail-safe regulatory system comprising two processes. First, HISE1 downregulates the protein levels of the β-hydroxy β-methylglutaryl-CoA reductases HMGR1 and HMGR2, which are rate-limiting enzymes in the sterol synthesis pathway, resulting in suppression of sterol overproduction. Second, if the first process is not successful, excess sterols are converted to sterol esters by phospholipid sterol acyltransferase1 (PSAT1) on ER microdomains and then segregated in SE bodies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
Sequence data from this study can be found in the GenBank/EMBL data libraries under the following accession numbers: CLO3 (At2g33380, NM_128898), HISE1 (At1g60995, NM_001084280), PSAT1 (At1g04010, NM_100282), HMGR1 (At1g76490, NM_106299), HMGR2 (At2g17370, NM_127292) and EF1a (At5g60390, NM_125432). The data that support the findings of this study are available from the corresponding author on request.
References
Ferrer, A., Altabella, T., Arro, M. & Boronat, A. Emerging roles for conjugated sterols in plants. Prog. Lipid. Res. 67, 27–37 (2017).
Banas, A. et al. Cellular sterol ester synthesis in plants is performed by an enzyme (phospholipid:sterol acyltransferase) different from the yeast and mammalian acyl-CoA:sterol acyltransferases. J. Biol. Chem. 280, 34626–34634 (2005).
Bouvier-Navé, P. et al. Involvement of the Phospholipid Sterol Acyltransferase1 in plant sterol homeostasis and leaf senescence. Plant Physiol. 152, 107–119 (2010).
Schaller, H. et al. Expression of the Hevea brasiliensis (H.B.K.) Mull. Arg. 3-hydroxy-3-methylglutaryl-coenzyme A reductase 1 in tobacco results in sterol overproduction. Plant Physiol. 109, 761–770 (1995).
Maillot-Vernier, P., Gondet, L., Schaller, H., Benveniste, P. & Belliard, G. Genetic study and further biochemical characterization of a tobacco mutant that overproduces sterols. Mol. Gen. Genet. 231, 33–40 (1991).
Gondet, L., Bronner, R. & Benveniste, P. Regulation of sterol content in membranes by subcellular compartmentation of steryl-esters accumulating in a sterol-overproducing tobacco mutant. Plant Physiol. 105, 509–518 (1994).
Wilkinson, S. C., Powls, R. & Goad, L. J. The effects of excess exogenous mevalonic acid on sterol and steryl ester biosynthesis in celery (Apium graveolens) cell suspension cultures. Phytochemistry 37, 1031–1035 (1994).
Diener, A. C. et al. STEROL METHYLTRANSFERASE 1 controls the level of cholesterol in plants. Plant Cell 12, 853–870 (2000).
Sonawane, P. D. et al. Plant cholesterol biosynthetic pathway overlaps with phytosterol metabolism. Nat. Plants 3, 16205 (2016).
Liu, J. & Nes, W. D. Steroidal triterpenes: design of substrate-based inhibitors of ergosterol and sitosterol synthesis. Molecules 14, 4690–4706 (2009).
Burg, J. S. & Espenshade, P. J. Regulation of HMG-CoA reductase in mammals and yeast. Prog. Lipid. Res. 50, 403–410 (2011).
Pollier, J. et al. The protein quality control system manages plant defence compound synthesis. Nature 504, 148–152 (2013).
Chye, M. L., Tan, C. T. & Chua, N. H. Three genes encode 3-hydroxy-3-methylglutaryl-coenzyme A reductase in Hevea brasiliensis: hmg1 and hmg3 are differentially expressed. Plant Mol. Biol. 19, 473–484 (1992).
Suzuki, M. et al. Loss of function of 3-hydroxy-3-methylglutaryl coenzyme A reductase 1 (HMG1) in Arabidopsis leads to dwarfing, early senescence and male sterility, and reduced sterol levels. Plant J. 37, 750–761 (2004).
Ohyama, K., Suzuki, M., Masuda, K., Yoshida, S. & Muranaka, T. Chemical phenotypes of the hmg1 and hmg2 mutants of Arabidopsis demonstrate the in-planta role of HMG-CoA reductase in triterpene biosynthesis. Chem. Pharm. Bull. 55, 1518–1521 (2007).
Ferrero, S. et al. Proliferation and morphogenesis of the endoplasmic reticulum driven by the membrane domain of 3-hydroxy-3-methylglutaryl coenzyme A reductase in plant cells. Plant Physiol. 168, 899–914 (2015).
Learned, R. M. & Fink, G. R. 3-Hydroxy-3-methylglutaryl-coenzyme A reductase from Arabidopsis thaliana is structurally distinct from the yeast and animal enzymes. Proc. Natl Acad. Sci. USA 86, 2779–2783 (1989).
Gardner, R. G., Shearer, A. G. & Hampton, R. Y. In vivo action of the HRD ubiquitin ligase complex: mechanisms of endoplasmic reticulum quality control and sterol regulation. Mol. Cell Biol. 21, 4276–4291 (2001).
Faulkner, R. A., Nguyen, A. D., Jo, Y. & DeBose-Boyd, R. A. Lipid-regulated degradation of HMG-CoA reductase and Insig-1 through distinct mechanisms in insect cells. J. Lipid Res. 54, 1011–1022 (2013).
Doblas, V. G. et al. The SUD1 gene encodes a putative E3 ubiquitin ligase and is a positive regulator of 3-hydroxy-3-methylglutaryl coenzyme a reductase activity in Arabidopsis. Plant Cell 25, 728–743 (2013).
Erffelinck, M. L. & Goossens, A. Endoplasmic reticulum-associated degradation (ERAD)-dependent control of (tri)terpenoid metabolism in plants. Planta Med. 84, 874–880 (2018).
Shimada, T. L. et al. Leaf oil body functions as a subcellular factory for the production of a phytoalexin in Arabidopsis. Plant Physiol. 164, 105–118 (2014).
Shimada, T. L., Takano, Y. & Hara-Nishimura, I. Oil body-mediated defense against fungi: From tissues to ecology. Plant Signal. Behav. 10, e989036 (2015).
Caelles, C., Ferrer, A., Balcells, L., Hegardt, F. G. & Boronat, A. Isolation and structural characterization of a cDNA encoding Arabidopsis thaliana 3-hydroxy-3-methylglutaryl coenzyme A reductase. Plant Mol. Biol. 13, 627–638 (1989).
Enjuto, M. et al. Arabidopsis thaliana contains two differentially expressed 3-hydroxy-3-methylglutaryl-CoA reductase genes, which encode microsomal forms of the enzyme. Proc. Natl Acad. Sci. USA 91, 927–931 (1994).
Kobayashi, K. et al. LOVASTATIN INSENSITIVE 1, a novel pentatricopeptide repeat protein, is a potential regulatory factor of isoprenoid biosynthesis in Arabidopsis. Plant Cell Physiol. 48, 322–331 (2007).
Kopischke, M. et al. Impaired sterol ester synthesis alters the response of Arabidopsis thaliana to Phytophthora infestans. Plant J. 73, 456–468 (2013).
Yang, B. et al. The critical role of membralin in postnatal motor neuron survival and disease. eLife 4, e06500 (2015).
Zhu, B. et al. ER-associated degradation regulates Alzheimer’s amyloid pathology and memory function by modulating γ-secretase activity. Nat. Commun. 8, 1472 (2017).
Tamura, K., Shimada, T., Kondo, M., Nishimura, M. & Hara-Nishimura, I. KATAMARI1/MURUS3 is a novel Golgi membrane protein that is required for endomembrane organization in Arabidopsis. Plant Cell 17, 1764–1776 (2005).
Shimada, T. L., Shimada, T., Takahashi, H., Fukao, Y. & Hara-Nishimura, I. A novel role for oleosins in freezing tolerance of oilseeds in Arabidopsis thaliana. Plant J. 55, 798–809 (2008).
Karimi, M., Inze, D. & Depicker, A. GATEWAY vectors for Agrobacterium-mediated plant transformation. Trends Plant Sci. 7, 193–195 (2002).
Shimada, T. L., Shimada, T. & Hara-Nishimura, I. A rapid and non-destructive screenable marker, FAST, for identifying transformed seeds of Arabidopsis thaliana. Plant J. 61, 519–528 (2010).
Nakamura, S. et al. Gateway binary vectors with the bialaphos resistance gene, bar, as a selection marker for plant transformation. Biosci. Biotechnol. Biochem. 74, 1315–1319 (2010).
Nakagawa, T. et al. Improved Gateway binary vectors: high-performance vectors for creation of fusion constructs in transgenic analysis of plants. Biosci. Biotechnol. Biochem. 71, 2095–2100 (2007).
Fujimoto, S., Sugano, S. S., Kuwata, K., Osakabe, K. & Matsunaga, S. Visualization of specific repetitive genomic sequences with fluorescent TALEs in Arabidopsis thaliana. J. Exp. Bot. 67, 6101–6110 (2016).
Shishido, Y. et al. A covalent G-site inhibitor for glutathione S-transferase Pi (GSTP1-1). Chem. Commun. 53, 11138–11141 (2017).
Tang, J., Kobayashi, K., Suzuki, M., Matsumoto, S. & Muranaka, T. The mitochondrial PPR protein LOVASTATIN INSENSITIVE 1 plays regulatory roles in cytosolic and plastidial isoprenoid biosynthesis through RNA editing. Plant J. 61, 456–466 (2010).
Maurey, K., Wolf, F. & Golbeck, J. 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in Ochromonas malhamensis: a system to study the relationship between enzyme activity and rate of steroid biosynthesis. Plant Physiol. 82, 523–527 (1986).
Bach, T. J., Rogers, D. H. & Rudney, H. Detergent-solubilization, purification, and characterization of membrane-bound 3-hydroxy-3-methylglutaryl-coenzyme A reductase from radish seedlings. Eur. J. Biochem. 154, 103–111 (1986).
Bligh, E. G. & Dyer, W. J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37, 911–917 (1959).
Bechtold, N. & Pelletier, G. In planta Agrobacterium-mediated transformation of adult Arabidopsis thaliana plants by vacuum infiltration. Methods Mol. Biol. 82, 259–266 (1998).
Kaido, M., Funatsu, N., Tsuno, Y., Mise, K. & Okuno, T. Viral cell-to-cell movement requires formation of cortical punctate structures containing Red clover necrotic mosaic virus movement protein. Virology 413, 205–215 (2011).
Mitsuhashi, N., Shimada, T., Mano, S., Nishimura, M. & Hara-Nishimura, I. Characterization of organelles in the vacuolar-sorting pathway by visualization with GFP in tobacco BY-2 cells. Plant Cell Physiol. 41, 993–1001 (2000).
Ueda, H. et al. Phosphorylation of the C terminus of RHD3 has a critical role in homotypic ER membrane fusion in Arabidopsis. Plant Physiol. 170, 867–880 (2016).
Uemura, T. et al. Qa-SNAREs localized to the trans-Golgi network regulate multiple transport pathways and extracellular disease resistance in plants. Proc. Natl Acad. Sci. USA 109, 1784–1789 (2012).
Nelson, B. K., Cai, X. & Nebenfuhr, A. A multicolored set of in vivo organelle markers for co-localization studies in Arabidopsis and other plants. Plant J. 51, 1126–1136 (2007).
Uemura, T. et al. Systematic analysis of SNARE molecules in Arabidopsis: dissection of the post-Golgi network in plant cells. Cell Struct. Funct. 29, 49–65 (2004).
Choi, S. W. et al. RABA members act in distinct steps of subcellular trafficking of the FLAGELLIN SENSING2 receptor. Plant Cell 25, 1174–1187 (2013).
Sparkes, I. A., Runions, J., Kearns, A. & Hawes, C. Rapid, transient expression of fluorescent fusion proteins in tobacco plants and generation of stably transformed plants. Nat. Protoc. 1, 2019–2025 (2006).
Brocard, L. et al. Proteomic analysis of lipid droplets from Arabidopsis aging leaves brings new insight into their biogenesis and functions. Front. Plant Sci. 8, 894 (2017).
Okazaki, Y. et al. A new class of plant lipid is essential for protection against phosphorus depletion. Nat. Commun. 4, 1510 (2013).
Okazaki, Y. & Saito, K. Plant lipidomics using UPLC–QTOF-MS. Methods Mol. Biol. 1778, 157–169 (2018).
Yamashita, K. et al. Use of novel picolinoyl derivatization for simultaneous quantification of six corticosteroids by liquid chromatography–electrospray ionization tandem mass spectrometry. J. Chromatogr. A. 1173, 120–128 (2007).
Okazaki, Y. et al. Induced accumulation of glucuronosyldiacylglycerol in tomato and soybean under phosphorus deprivation. Physiol. Plant. 155, 33–42 (2015).
Acknowledgements
We thank T. Nakagawa (Shimane University), S. Ishiguro (Nagoya University), M. Kaido (Kyoto University), T. Uemura (University of Tokyo), A. Nebenführ (University of Tennessee), E. Ito (International Christian University), K. Ebine (National Institute for Basic Biology), T. Goh (Nara Institute of Science and Technology) and Plant System Biology (VIB) for their donations of the vectors; S. Arai (Ochanomizu University), R. Iwahori (Ochanomizu University) and K. Takano (RIKEN Center for Sustainable Resource Science) for their technical assistance; the Arabidopsis Biological Resource Center for providing seeds of A. thaliana T-DNA insertion mutants; the Model Plant Research Facility (NIBB BioResource Center) and the Japan Advanced Plant Science Network (RIKEN) for their technical support; T. Muranaka (Osaka University) for the HMG1cd antibody; M. Hanaoka (Chiba University) for real-time PCR analysis; and J. Raymond (Eigoken) for critical readings of this manuscript. This work was supported by Grants-in-Aid for Scientific Research to I.H.-N. (no. 15H05776 and 22000014) and to T.L.S. (no. 16K18834 and 19K05809) from the Japan Society for the Promotion of Science (JSPS), by Leading Initiative for Excellent Young Researchers (LEADER) to T.L.S. (no. J16HJ00026) from the Ministry of Education, Culture, Sports, Science and Technology in Japan (MEXT), by SUNBOR GRANT of Suntory Foundation for life science to T.L.S., by Kato Memorial Bioscience Foundation to T.L.S., by Phytochemical Plant Molecular Science of Strategic Priority Research Promotion Program to T.L.S. and K.S. from Chiba University, and by the Hirao Taro Foundation of KONAN GAKUEN for Academic Research to I.H.-N.
Author information
Authors and Affiliations
Contributions
T.L.S., T.S. and I.H.-N. designed the research. T.L.S. performed the experiments, except for lipidome, proteome and radiolabelling experiments. Y.O., Y.H. and K.S. performed lipidome experiments, K.K. performed proteome experiments, and K.O. and M.K. performed in vivo- and in vitro-radiolabelling experiments. H.U. contributed to cell biological analysis and A.N., T.U. and Y.T. contributed to discussions. T.L.S. and I.H.-N. analysed the data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Plants thanks Narciso Campos, Hubert Schaller and the other, anonymous, reviewers for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figures and Supplementary Tables.
Rights and permissions
About this article
Cite this article
Shimada, T.L., Shimada, T., Okazaki, Y. et al. HIGH STEROL ESTER 1 is a key factor in plant sterol homeostasis. Nat. Plants 5, 1154–1166 (2019). https://doi.org/10.1038/s41477-019-0537-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-019-0537-2
This article is cited by
-
Genomic analysis of an ultrasmall freshwater green alga, Medakamo hakoo
Communications Biology (2023)
-
A fungal tolerance trait and selective inhibitors proffer HMG-CoA reductase as a herbicide mode-of-action
Nature Communications (2022)
-
Excess sterols disrupt plant cellular activity by inducing stress-responsive gene expression
Journal of Plant Research (2020)
-
Unleashed sterol production in thale cress
Nature Plants (2019)