Abstract
In nature, plants experience large fluctuations in light intensity and they need to balance the absorption and utilization of this energy appropriately. Non-photochemical quenching (NPQ) is a rapidly switchable mechanism that protects plants from photodamage caused by high light exposure by dissipating the excess absorbed energy as heat. It is triggered by the pH gradient across the thylakoid membrane and requires the protein PsbS and the xanthophyll zeaxanthin. However, the site and mechanism of the quencher(s) remain unknown. Here, we constructed a mutant of Arabidopsis thaliana that lacks light-harvesting complex II (LHCII), the main antenna complex of plants, to verify its contribution to NPQ. The mutant plant has normally stacked thylakoid membranes, displays no upregulation of other LHCs but shows a relative decrease in Photosystem I (PSI), which compensates for the decrease of the PSII antenna. The mutant plant exhibits a reduction in NPQ of about 60% and the remaining NPQ resembles that of mutant plants lacking chlorophyll (Chl) b, which lack all PSII peripheral antenna complexes. We thus report that PsbS-dependent NPQ occurs mainly in LHCII, but there is an additional quenching site in the PSII core.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in the published article and its Supplementary Information.
References
Su, X. et al. Structure and assembly mechanism of plant C2S2M2-type PSII–LHCII supercomplex. Science 357, 815–820 (2017).
Barber, J. Photosystem II: the water splitting enzyme of photosynthesis and the origin of oxygen in our atmosphere. Q. Rev. Biophys. 49, e14 (2016).
Nicol, L. & Croce, R. in Light Harvesting in Photosynthesis (eds Croce, R. et al.) 73–90 (CRC Press, 2018).
Caffarri, S., Croce, R., Cattivelli, L. & Bassi, R. A look within LHCII: differential analysis of the Lhcb1–3 complexes building the major trimeric antenna complex of higher-plant photosynthesis. Biochemistry 43, 9467–9476 (2004).
Kouřil, R., Wientjes, E., Bultema, J. B., Croce, R. & Boekema, E. J. High-light vs. low-light: Effect of light acclimation on Photosystem II composition and organization in Arabidopsis thaliana. Biochim. Biophys. Acta 1827, 411–419 (2013).
Croce, R. & van Amerongen, H. Natural strategies for photosynthetic light harvesting. Nat. Chem. Biol. 10, 492–501 (2014).
Powles, S. B. Photoinhibition of photosynthesis induced by visible Light. Annu. Rev. Plant Physiol. 35, 15–44 (1984).
Ruban, A. V., Johnson, M. P. & Duffy, C. D. P. The photoprotective molecular switch in the photosystem II antenna. Biochim. Biophys. Acta 1817, 167–181 (2012).
Briantais, J.-M., Vernotte, C., Picaud, M. & Krause, G. H. A quantitative study of the slow decline of chlorophyll a fluorescence in isolated chloroplasts. Biochim. Biophys. Acta 548, 128–138 (1979).
Li, X.-P., Muller-Moule, P., Gilmore, A. M. & Niyogi, K. K. PsbS-dependent enhancement of feedback de-excitation protects photosystem II from photoinhibition. Proc. Natl Acad. Sci. USA 99, 15222–15227 (2002).
Li, X.-P. et al. Regulation of photosynthetic light harvesting involves intrathylakoid lumen pH sensing by the PsbS protein. J. Biol. Chem. 279, 22866–22874 (2004).
Yamamoto, H. Y., Nakayama, T. O. M. & Chichester, C. O. Studies on the light and dark interconversions of leaf xanthophylls. Arch. Biochem. Biophys. 97, 168–173 (1962).
Demmig-Adams, B. Carotenoids and photoprotection in plants: a role for the xanthophyll zeaxanthin. Biochim. Biophys. Acta 1020, 1–24 (1990).
Dominici, P. et al. Biochemical properties of the PsbS subunit of photosystem II either purified from chloroplast or recombinant. J. Biol. Chem. 277, 22750–22758 (2002).
Fan, M. et al. Crystal structures of the PsbS protein essential for photoprotection in plants. Nat. Struct. Mol. Biol. 22, 729–735 (2015).
Holt, N. E. et al. Carotenoid cation formation and the regulation of photosynthetic light harvesting. Science 307, 433–436 (2005).
Horton, P. et al. Control of the light-harvesting function of chloroplast membranes by aggregation of the LHCII chlorophyll–protein complex. FEBS Lett. 292, 1–4 (1991).
Horton, P., Ruban, A. V. & Walters, R. G. Regulation of light harvesting in green plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 47, 655–684 (1996).
Horton, P., Ruban, A. V. & Wentworth, M. Allosteric regulation of the light-harvesting system of Photosystem II. Philos. Trans. R. Soc. Lond. B. 355, 1361–1370 (2000).
Ruban, A. V., Phillip, D., Young, A. J. & Horton, P. Carotenoid-dependent oligomerization of the major chlorophyll a/b light harvesting complex of Photosystem II of plants. Biochemistry 36, 7855–7859 (1997).
Johnson, M. P. et al. Photoprotective energy dissipation involves the reorganization of Photosystem II light-harvesting complexes in the grana membranes of spinach chloroplasts. Plant Cell 23, 1468–1479 (2011).
Goral, T. K. et al. Light-harvesting antenna composition controls the macrostructure and dynamics of thylakoid membranes in Arabidopsis. Plant J. 69, 289–301 (2012).
Walters, R. G., Ruban, A. V. & Horton, P. Higher plant light-harvesting complexes LHCIIa and LHCIIc are bound by dicyclohexylcarbodiimide during inhibition of energy dissipation. Eur. J. Biochem. 226, 1063–1069 (1994).
Morosinotto, T., Baronio, R. & Bassi, R. Dynamics of chromophore binding to Lhc proteins in vivo and in vitro during operation of the xanthophyll cycle. J. Biol. Chem. 277, 36913–36920 (2002).
Pesaresi, P., Sandonà, D., Giuffra, E. & Bassi, R. A single point mutation (E166Q) prevents dicyclohexylcarbodiimide binding to the photosystem II subunit CP29. FEBS Lett. 402, 151–156 (1997).
Holzwarth, A. R., Miloslavina, Y., Nilkens, M. & Jahns, P. Identification of two quenching sites active in the regulation of photosynthetic light-harvesting studied by time-resolved fluorescence. Chem. Phys. Lett. 483, 262–267 (2009).
de Bianchi, S., Dall’Osto, L., Tognon, G., Morosinotto, T. & Bassi, R. Minor antenna proteins CP24 and CP26 affect the interactions between Photosystem II subunits and the electron transport rate in grana membranes of Arabidopsis. Plant Cell 20, 1012–1028 (2008).
de Bianchi, S. et al. Arabidopsis mutants deleted in the light-harvesting protein Lhcb4 have a disrupted Photosystem II macrostructure and are defective in photoprotection. Plant Cell 23, 2659–2679 (2011).
Andersson, J. et al. Absence of the Lhcb1 and Lhcb2 proteins of the light-harvesting complex of Photosystem II—effects on photosynthesis, grana stacking and fitness. Plant J. 35, 350–361 (2003).
Dall’Osto, L., Ünlü, C., Cazzaniga, S. & van Amerongen, H. Disturbed excitation energy transfer in Arabidopsis thaliana mutants lacking minor antenna complexes of Photosystem II. Biochim. Biophys. Acta 1837, 1981–1988 (2014).
Ruban, A. V. et al. Plants lacking the main light-harvesting complex retain Photosystem II macro-organization. Nature 421, 648–652 (2003).
Pietrzykowska, M. et al. The light-harvesting chlorophyll a/b binding proteins Lhcb1 and Lhcb2 play complementary roles during state transitions in Arabidopsis. Plant Cell 26, 3646–3660 (2014).
Jackowski, G., Kacprzak, K. & Jansson, S. Identification of Lhcb1/Lhcb2/Lhcb3 heterotrimers of the main light-harvesting chlorophyll a/b–protein complex of Photosystem II (LHC II). Biochim. Biophys. Acta 1504, 340–345 (2001).
Lazár, D. & Pospíšil, P. Mathematical simulation of chlorophyll a fluorescence rise measured with 3-(3′,4′-dichlorophenyl)-1,1-dimethylurea-treated barley leaves at room and high temperatures. Eur. Biophys. J. 28, 468–477 (1999).
Tian, L. et al. pH dependence, kinetics and light-harvesting regulation of nonphotochemical quenching in Chlamydomonas. Proc. Natl Acad. Sci. USA 116, 8320–8325 (2019).
Bailleul, B., Cardol, P., Breyton, C. & Finazzi, G. Electrochromism: a useful probe to study algal photosynthesis. Photosynth. Res. 106, 179–189 (2010).
Dekker, J. P. & Boekema, E. J. Supramolecular organization of thylakoid membrane proteins in green plants. Biochim. Biophys. Acta 1706, 12–39 (2005).
van der Weij-de Wit, C. D., Ihalainen, J. A., van Grondelle, R. & Dekker, J. P. Excitation energy transfer in native and unstacked thylakoid membranes studied by low temperature and ultrafast fluorescence spectroscopy. Photosynth. Res. 93, 173–182 (2007).
Bassi, R., Hinz, U. & Barbato, R. The role of the light harvesting complex and Photosystem II in thylakoid stacking in the chlorina-f2 barley mutant. Carlsberg Res. Commun. 50, 347–367 (1985).
Kim, E.-H. et al. The multiple roles of light-harvesting chlorophyll a/b-protein complexes define structure and optimize function of Arabidopsis chloroplasts: a study using two chlorophyll b-less mutants. Biochim. Biophys. Acta 1787, 973–984 (2009).
Niyogi, K. K., Grossman, A. R. & Björkman, O. Arabidopsis mutants define a central role for the xanthophyll cycle in the regulation of photosynthetic energy conversion. Plant Cell 10, 1121–3418 (1998).
Espineda, C. E., Linford, A. S., Devine, D. & Brusslan, J. A. The AtCAO gene, encoding chlorophyll a oxygenase, is required for chlorophyll b synthesis in Arabidopsis thaliana. Proc. Natl Acad. Sci. USA 96, 10507–10511 (1999).
Havaux, M., Dall’osto, L. & Bassi, R. Zeaxanthin has enhanced antioxidant capacity with respect to all other xanthophylls in Arabidopsis leaves and functions independent of binding to PSII antennae. Plant Physiol. 145, 1506–1520 (2007).
Siefermann, D. & Yamamoto, H. Y. NADPH and oxygen-dependent epoxidation of zeaxanthin in isolated chloroplasts. Biochem. Biophys. Res. Commun. 62, 456–461 (1975).
Yamamoto, H. Y. & Kamite, L. The effects of dithiothreitol on violaxanthin de-epoxidation and absorbance changes in the 500-nm region. Biochim. Biophys. Acta 267, 538–543 (1972).
Johnson, M. P., Pérez-Bueno, M. L., Zia, A., Horton, P. & Ruban, A. V. The zeaxanthin-independent and zeaxanthin-dependent qE components of nonphotochemical quenching involve common conformational changes within the Photosystem II antenna in Arabidopsis. Plant Physiol. 149, 1061–1075 (2009).
Wraight, C. A. & Crofts, A. R. Energy-dependent quenching of chlorophyll a fluorescence in isolated chloroplasts. Eur. J. Biochem. 17, 319–327 (1970).
Murata, N. & Sugahara, K. Light-induced decrease of chlorophyll a fluorescence related to photophosphorylation system in spinach chloroplasts. Biochim. Biophys. Acta 189, 182–192 (1969).
Ruban, A. V. Nonphotochemical chlorophyll fluorescence quenching: mechanism and effectiveness in protecting plants from photodamage. Plant Physiol. 170, 1903–1916 (2016).
Avenson, T. J. et al. Zeaxanthin radical cation formation in minor light-harvesting complexes of higher plant antenna. J. Biol. Chem. 283, 3550–3558 (2008).
Dall’Osto, L. et al. Two mechanisms for dissipation of excess light in monomeric and trimeric light-harvesting complexes. Nat. Plants 3, 17033 (2017).
Farooq, S. et al. Dynamic feedback of the Photosystem II reaction centre on photoprotection in plants. Nat. Plants 4, 225–231 (2018).
Li, X.-P. et al. A pigment-binding protein essential for regulation of photosynthetic light harvesting. Nature 403, 391–395 (2000).
Correa-Galvis, V., Poschmann, G., Melzer, M., Stühler, K. & Jahns, P. PsbS interactions involved in the activation of energy dissipation in Arabidopsis. Nat. Plants 2, 15225 (2016).
Townsend, A. J. et al. The causes of altered chlorophyll fluorescence quenching induction in the Arabidopsis mutant lacking all minor antenna complexes. Biochim. Biophys. Acta 1859, 666–675 (2018).
Xu, P., Tian, L., Kloz, M. & Croce, R. Molecular insights into zeaxanthin-dependent quenching in higher plants. Sci. Rep. 5, 13679 (2015).
Schägger, H. Tricine–SDS–PAGE. Nat. Protoc. 1, 16–22 (2006).
Järvi, S., Suorsa, M., Paakkarinen, V. & Aro, E.-M. Optimized native gel systems for separation of thylakoid protein complexes: novel super-and mega-complexes. Biochem. J. 439, 207–214 (2011).
Croce, R., Canino, G., Ros, F. & Bassi, R. Chromophore organization in the higher-plant Photosystem II antenna protein CP26. Biochemistry 41, 7334–7343 (2002).
Fristedt, R. et al. RBF1, a plant homolog of the bacterial ribosome-binding factor RbfA, acts in processing of the chloroplast 16S ribosomal RNA. Plant Physiol. 164, 201–215 (2014).
Rueden, C. T. et al. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinformatics 18, 529 (2017).
Wientjes, E. & Croce, R. The light-harvesting complexes of higher-plant Photosystem I: Lhca1/4 and Lhca2/3 form two red-emitting heterodimers. Biochem. J. 433, 477–485 (2011).
Acknowledgements
We thank L. Roy for constructing the knockdown line, S. Jansson for the gift of the amiLhcb1 and amiLhcb2 seeds, A. Rubert Albiol for help in selecting the knockdown line and H. van Amerongen for helpful discussion. Electron microscopy was performed at the Vrije Universiteit Electron Microscopy Facility. R.C. received financial support from the Netherlands Organization for Scientific Research (NWO) (86510013) and the European Commission (EC) (214113). W.J.N. was supported by a European Commission Marie Curie Actions Individual Fellowship (799083). L.N. received financial support from the New Zealand Government through the Royal Society of New Zealand–Rutherford Foundation.
Author information
Authors and Affiliations
Contributions
R.C. conceived the project. L.N. and W.J.N. performed the experiments. Data were analysed and interpreted by L.N., W.J.N. and R.C. The manuscript was written by L.N. with contributions by W.J.N. and R.C.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer Review Information Nature Plants thanks Peter Jahns and the other, anonymous, reviewers for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Identity of Band 4 present in NoLHCII.
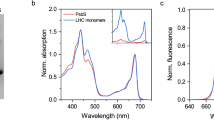
a, Sucrose gradients of WT and NoLHCII thylakoids. b, Coomassie-stained SDS-PAGE of NoLHCII sucrose bands with WT thylakoids, band 3 and 4 loaded as controls. c-d, Absorption spectra of bands 3 and 4 from WT and NoLHCII, normalized to the Qy maximum. Note the increased Chl a/b ratio of the NoLHCII samples e-f, 77 K fluorescence emission spectra of bands 3 and 4 from WT and NoLHCII excited at 440 nm. Spectra are normalized to their maximum value. Note the appearance of a band peaking around 730 nm in NoLHCII samples. This indicates the presence of Lhca’s, as the two functional heterodimers, Lhca1/4 and Lhca2/3, both emit in this region, with a maximum around 730 nm at 77 K62. a-f, Experiments were repeated independently, twice with similar results.
Extended Data Fig. 2 Distribution of granal width and height in WT (a,c) and NoLHCII (b,d) thylakoids.
Transmission electron microscopy was performed on transverse sections of dark-adapted 5-week-old Arabidopsis leaves. The number of grana in each size class is presented as a percentage of the total number of grana measured. This was 203 and 235 for WT and NoLHCII, respectively. According to Student’s t-test (two-tailed), there is no significant difference in grana width; t(438) = - 1.76, p = 0.08, however there is a small but significant decrease in grana height in NoLHCII (0.09 ± 0.04) compared to WT (0.11 ± 0.05); t(438) = 4.46, p = 1.06E-5. Data represent the mean ± s.d. (n= 3 three biologically independent samples).
Extended Data Fig. 3 Carotenoid composition of WT and NoLHCII leaves normalised to 100 Chl a.
1. The data represent the mean ± s.d (n = 3 biologically independent samples) 2. A, antheraxanthin; N, neoxanthin; V, violaxanthin; L, lutein; Z, zeaxanthin; β-C, β-carotene.
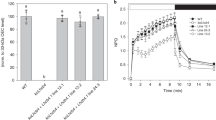
Extended Data Fig. 4 Zeaxanthin dependence of NPQ. Leaves from WT and NoLHCII were vacuum infiltrated in a Hepes-sorbitol (HS) solution with and without the presence of 5 mM DTT.
a, NPQ was induced using an illumination intensity of 1287 μmol photons m−2 s−1. The data represent the mean ± s.d (n = 3 biologically independent experiments). b, Pigment analysis of DTT infiltrated leaves via HPLC shows the absence of zeaxanthin following illumination with 1287 μmol photons m−2 s−1 for 30 minutes. This experiment was repeated independently three times with similar results.
Supplementary information
Supplementary Information
Supplementary Figs. 1–3 and Supplementary Table 1.
Source data
Rights and permissions
About this article
Cite this article
Nicol, L., Nawrocki, W.J. & Croce, R. Disentangling the sites of non-photochemical quenching in vascular plants. Nat. Plants 5, 1177–1183 (2019). https://doi.org/10.1038/s41477-019-0526-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-019-0526-5
This article is cited by
-
The nature of carotenoid S* state and its role in the nonphotochemical quenching of plants
Nature Communications (2024)
-
Thylakoid ultrastructural variations in chlorophyll-deficient wheat: aberrations or structural acclimation?
Planta (2024)
-
Cryo-EM structures of LHCII in photo-active and photo-protecting states reveal allosteric regulation of light harvesting and excess energy dissipation
Nature Plants (2023)
-
Recent progress in atomistic modeling of light-harvesting complexes: a mini review
Photosynthesis Research (2023)
-
A perspective on the major light-harvesting complex dynamics under the effect of pH, salts, and the photoprotective PsbS protein
Photosynthesis Research (2023)