Abstract
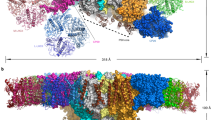
In plants and green algae, the core of photosystem I (PSI) is surrounded by a peripheral antenna system consisting of light-harvesting complex I (LHCI). Here we report the cryo-electron microscopic structure of the PSI–LHCI supercomplex from the green alga Chlamydomonas reinhardtii. The structure reveals that eight Lhca proteins form two tetrameric LHCI belts attached to the PsaF side while the other two Lhca proteins form an additional Lhca2/Lhca9 heterodimer attached to the opposite side. The spatial arrangement of light-harvesting pigments reveals that Chlorophylls b are more abundant in the outer LHCI belt than in the inner LHCI belt and are absent from the core, thereby providing the downhill energy transfer pathways to the PSI core. PSI–LHCI is complexed with a plastocyanin on the patch of lysine residues of PsaF at the luminal side. The assembly provides a structural basis for understanding the mechanism of light-harvesting, excitation energy transfer of the PSI–LHCI supercomplex and electron transfer with plastocyanin.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
Atomic coordinates of the green algal PSI–LHCI have been deposited in the Protein Data Bank with accession codes 6JO5 (PSI–10Lhca) and 6JO6 (PSI–8Lhca). Cryo-EM maps of green algal PSI–LHCI have been deposited in the Electron Microscopy Data Bank with accession codes EMD-9853 (map c2), EMD-9854 (map d), EMD-9855 (map a) and EMD-9856 (map b).
References
Nelson, N. & Junge, W. Structure and energy transfer in photosystems of oxygenic photosynthesis. Annu. Rev. Biochem. 84, 659–683 (2015).
Suga, M., Qin, X., Kuang, T. & Shen, J. R. Structure and energy transfer pathways of the plant photosystem I-LHCI supercomplex. Curr. Opin. Struct. Biol. 39, 46–53 (2016).
Nelson, N. Plant photosystem I - the most efficient nano-photochemical machine. J. Nanosci. Nanotechnol. 9, 1709–1713 (2009).
Hippler, M., Drepper, F., Haehnel, W. & Rochaix, J. D. The N-terminal domain of PsaF: precise recognition site for binding and fast electron transfer from cytochrome c6 and plastocyanin to photosystem I of Chlamydomonas reinhardtii. Proc. Natl Acad. Sci. USA 95, 7339–7344 (1998).
Hippler, M., Drepper, F., Rochaix, J. D. & Muhlenhoff, U. Insertion of the N-terminal part of PsaF from Chlamydomonas reinhardtii into photosystem I from Synechococcus elongatus enables efficient binding of algal plastocyanin and cytochrome c6. J. Biol. Chem. 274, 4180–4188 (1999).
Kubota-Kawai, H. et al. X-ray structure of an asymmetrical trimeric ferredoxin–photosystem I complex. Nat. Plants 4, 218–224 (2018).
Blankenship, R. E. Origin and early evolution of photosynthesis. Photosynth. Res. 33, 91–111 (1992).
Jordan, P. et al. Three-dimensional structure of cyanobacterial photosystem I at 2.5 A resolution. Nature 411, 909–917 (2001).
Mazor, Y., Borovikova, A., Caspy, I. & Nelson, N. Structure of the plant photosystem I supercomplex at 2.6 A resolution. Nat. Plants 3, 17014 (2017).
Qin, X., Suga, M., Kuang, T. & Shen, J. R. Structural basis for energy transfer pathways in the plant PSI-LHCI supercomplex. Science 348, 989–995 (2015).
Mazor, Y., Borovikova, A. & Nelson, N. The structure of plant photosystem I super-complex at 2.8 A resolution. eLife 4, e07433 (2015).
Pan, X. W., Liu, Z. F., Li, M. & Chang, W. R. Architecture and function of plant light-harvesting complexes II. Curr. Opin. Struct. Biol. 23, 515–525 (2013).
Drop, B. et al. Photosystem I of Chlamydomonas reinhardtii contains nine light-harvesting complexes (Lhca) located on one side of the core. J. Biol. Chem. 286, 44878–44887 (2011).
Ozawa, S. et al. Configuration of ten light-harvesting chlorophyll a/b complex I subunits in Chlamydomonas reinhardtii photosystem I. Plant Physiol. 178, 583–595 (2018).
Kubota-Kawai, H. et al. Ten antenna proteins are associated with the core in the supramolecular organization of the photosystem I supercomplex in Chlamydomonas reinhardtii. J. Biol. Chem. 294, 4304–4314 (2019).
Su, X. et al. Antenna arrangement and energy transfer pathways of a green algal photosystem-I-LHCI supercomplex. Nat. Plants 5, 273–281 (2019).
Chen, V. B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D 66, 12–21 (2010).
Watanabe, A., Kim, E., Burton-Smith, R. N., Tokutsu, R. & Minagawa, J. Amphipol-assisted purification method for the highly active and stable photosystem II supercomplex of Chlamydomonas reinhardtii. FEBS Lett. 593, 1072–1079 (2019).
Ozawa, S., Kosugi, M., Kashino, Y., Sugimura, T. & Takahashi, Y. 5′-Monohydroxyphylloquinone is the dominant naphthoquinone of PSI in the green alga Chlamydomonas reinhardtii . Plant Cell Physiol. 53, 237–243 (2012).
Liu, Z. et al. Crystal structure of spinach major light-harvesting complex at 2.72 A resolution. Nature 428, 287–292 (2004).
Pan, X. et al. Structural insights into energy regulation of light-harvesting complex CP29 from spinach. Nat. Struct. Mol. Biol. 18, 309–315 (2011).
Wei, X. et al. Structure of spinach photosystem II-LHCII supercomplex at 3.2 A resolution. Nature 534, 69–74 (2016).
Bujaldon, S. et al. Functional accumulation of antenna proteins in chlorophyll b-less mutants of Chlamydomonas reinhardtii. Mol. Plant 10, 115–130 (2017).
Qin, X. et al. Structure of a green algal photosystem I in complex with a large number of light-harvesting complex I subunits. Nat. Plants 5, 263–272 (2019).
Fan, M. et al. Crystal structures of the PsbS protein essential for photoprotection in plants. Nat. Struct. Mol. Biol. 22, 729–735 (2015).
Steinbeck, J. et al. Structure of a PSI-LHCI-cyt b6f supercomplex in Chlamydomonas reinhardtii promoting cyclic electron flow under anaerobic conditions. Proc. Natl Acad. Sci. USA 115, 10517–10522 (2018).
Pi, X. et al. Unique organization of photosystem I-light-harvesting supercomplex revealed by cryo-EM from a red alga. Proc. Natl Acad. Sci. USA 115, 4423–4428 (2018).
Pan, X. et al. Structure of the maize photosystem I supercomplex with light-harvesting complexes I and II. Science 360, 1109–1113 (2018).
Shimada, S. et al. Complex structure of cytochrome c-cytochrome c oxidase reveals a novel protein-protein interaction mode. EMBO J. 36, 291–300 (2016).
Fischer, N., Stampacchia, O., Redding, K. & Rochaix, J. D. Selectable marker recycling in the chloroplast. Mol. Gen. Genet. 251, 373–380 (1996).
Kuroda, H., Kodama, N., Sun, X. Y., Ozawa, S. & Takahashi, Y. Requirement for Asn298 on D1 protein for oxygen evolution: analyses by exhaustive amino acid substitution in the green alga Chlamydomonas reinhardtii. Plant Cell Physiol. 55, 1266–1275 (2014).
Ozawa, S. et al. Biochemical and structural studies of the large Ycf4-photosystem I assembly complex of the green alga Chlamydomonas reinhardtii. Plant Cell 21, 2424–2442 (2009).
Chua, N. H. & Bennoun, P. Thylakoid membrane polypeptides of Chlamydomonas reinhardtii: wild-type and mutant strains deficient in photosystem II reaction center. Proc. Natl Acad. Sci. USA 72, 2175–2179 (1975).
Fling, S. P. & Gregerson, D. S. Peptide and protein molecular weight determination by electrophoresis using a high-molarity Tris buffer system without urea. Anal. Biochem. 155, 83–88 (1986).
Ozawa, S., Onishi, T. & Takahashi, Y. Identification and characterization of an assembly intermediate subcomplex of photosystem I in the green alga Chlamydomonas reinhardtii. J. Biol. Chem. 285, 20072–20079 (2010).
Yamano, T. et al. Light and low-CO2-dependent LCIB-LCIC complex localization in the chloroplast supports the carbon-concentrating mechanism in Chlamydomonas reinhardtii. Plant Cell Physiol. 51, 1453–1468 (2010).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Zhang, K. Gctf: real-time CTF determination and correction. J. Struct. Biol. 193, 1–12 (2016).
Scheres, S. H. RELION: implementation of a Bayesian approach to cryo-EM structure determination. J. Struct. Biol. 180, 519–530 (2012).
Pettersen, E. F. et al. UCSF chimera–a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D 60, 2126–2132 (2004).
Adams, P. D. et al. PHENIX: a comprehensive python-based system for macromolecular structure solution. Acta Crystallogr. D 66, 213–221 (2010).
Acknowledgements
We thank J.-R. Shen and K. Iwasaki for discussions, especially at the initial stage of the project. We also thank A. Watanabe, R. Tokutsu and J. Minagawa at the National Institute for Basic Biology for the valuable suggestion to use Amphipol in the PSI-LHCI preparations. This research was supported by the Platform Project for Supporting Drug Discovery and Life Science Research (Basis for Supporting Innovative Drug Discovery and Life Science Research) from AMED. This work was supported by JSPS KAKENHI (grant nos. JP16H06162 and JP16H06296 to M.S. and JP16H06554 to Y.T.). This work was also supported by JST, PREST (grant no. JP18069982 to M.S.).
Author information
Authors and Affiliations
Contributions
M.S., N.M. and Y.T. conceived the project. S.-I.O. and K.Y.-M. prepared the samples. N.M. and F.A. collected the cryo-EM data. N.M. processed the cryo-EM data. M.S. built and refined the structure. M.S., S.-I.O. and Y.T. wrote the manuscript. All authors discussed and commented on the results and the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer Review Information: Nature Plants thanks Alexey Amunts, Jean-David Rochaix and other anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–13, Supplementary Tables 1–7.
Rights and permissions
About this article
Cite this article
Suga, M., Ozawa, SI., Yoshida-Motomura, K. et al. Structure of the green algal photosystem I supercomplex with a decameric light-harvesting complex I. Nat. Plants 5, 626–636 (2019). https://doi.org/10.1038/s41477-019-0438-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-019-0438-4
This article is cited by
-
Architecture of symbiotic dinoflagellate photosystem I–light-harvesting supercomplex in Symbiodinium
Nature Communications (2024)
-
Uncovering the photosystem I assembly pathway in land plants
Nature Plants (2024)
-
Uphill energy transfer mechanism for photosynthesis in an Antarctic alga
Nature Communications (2023)
-
Structural insights into a unique PSI–LHCI–LHCII–Lhcb9 supercomplex from moss Physcomitrium patens
Nature Plants (2023)
-
Structural insights into the assembly and energy transfer of the Lhcb9-dependent photosystem I from moss Physcomitrium patens
Nature Plants (2023)