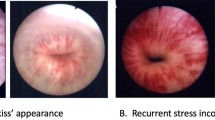
Abstract
The Rigicon ContiClassic® is a new sphincteric device designed to treat male stress urinary incontinence. This study evaluates the surgical outcomes and safety profile of the first 116 patients who received the implant between September 2021 and April 2022. Data were collected from patient information forms completed at the time of the implant and submitted by implanting surgeons, nursing staff in the Operating Room or company representatives present during the surgery. The study analyzed patient demographics, surgical details, and etiology of incontinence. The mean age of patients was 68.3 years +/− 9.65 yrs. Minimum age was 23 and maximum age was 83. The most common reason for implantation was urinary incontinence (58.6%) after radical prostatectomy. The results showed a revision rate of 6.90%, with three cases of fluid loss, four cases of iatrogenic mistaken sizing, and one case of patient dissatisfaction. There were no reported infections. Kaplan-Meier calculation showed survival rate of 93.2% at 12 months. This study shows the early safety outcomes for the Rigicon ContiClassic® sphincter device to be comparable to others presently on the market.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 8 print issues and online access
$259.00 per year
only $32.38 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request.
References
Sandhu JS, Breyer B, Comiter C, Eastham JA, Gomez C, Kirages DJ, et al. Incontinence after prostate treatment: AUA/SUFU guideline. J Urol. 2019;202:369–78.
Wright JL, Nathens AB, Rivara FP, Mackenzie EJ, Wessells H. Specific fracture configurations predict sexual and excretory dysfunction in men and women 1 year after pelvic fracture. J Urol. 2006;176:1540–5.
Koch GE, Kaufman MR. Male stress urinary incontinence. Urol Clin North Am. 2022;49:403–18.
Pizzol D, Demurtas J, Celotto S, Maggi S, Smith L, Angiolelli G, et al. Urinary incontinence and quality of life: a systematic review and meta-analysis. Aging Clin Exp Res. 2022;33:25–35.
Chung E. Contemporary surgical devices for male stress urinary incontinence: a review of technological advances in current continence surgery. Trans Androl Urol. 2017;6:S112–21.
Fuller TW, Ballon-Landa E, Gallo K, Smith TG 3rd, Ajay D, Westney OL, et al. Outcomes and Risk Factors of Revision and Replacement Artificial Urinary Sphincter Implantation in Radiated and Nonradiated Cases. J Urol. 2020;204:110–14.
Wilson SK, Westney OL, Mulcahy JJ. Twenty years later: is the scrotal one-incision AUS of value? Int J Impot Res. 2022;34:243–51.
Terlecki RP, Wilson SK. A new paradigm for surgical revision of the artificial urinary sphincter for recurrent stress urinary incontinence. Int J Impot Res. 2022;34:37–43.
Wilson S, Delk J 2nd, Henry GD, Siegel AL. New surgical technique for sphincter urinary control system using upper transverse scrotal incision. J Urol. 2003;169:261–64.
Abrams P, Constable LD, Cooper D, MacLennan G, Drake MJ, Harding C, et al. Outcomes of a noninferiority randomized controlled trial of surgery for men with urodynamic stress incontinence after prostate surgery (MASTER). Eur Urol. 2021;79:812–23.
Yoon PD, Chalasani V, Woo HH. Use of Clavien-Dindo classification in reporting and grading complications after urological surgical procedures: analysis of 2010-2012. J Urol. 2013;190:1271–4.
Scott FB, Bradley WE, Timm GW. Treatment of urinary incontinence by implantable prosthetic sphincter. Urology. 1973;1:252–59.
Wilson SK, Wen L, Rossello M, Maria P, Carrion R, Perito P, et al. Initial safety outcomes for the Rigicon Infla10® inflatable penile prosthesis. published online ahead of print, 2023 BJU Int. https://doi.org/10.1111/bju.15960.
Carson CC, Mulcahy JJ, Govier FE. Efficacy, safety and patient satisfaction outcomes of the AMS 700CX inflatable penile prosthesis: results of a long-term multicenter study. AMS 700CX Study Group. J Urol. 2000;164:376–80.
Goldstein I, Newman L, Baum N, Brooks M, Chaikin L, Goldberg K, et al. Safety and efficacy outcome of mentor alpha-1 inflatable penile prosthesis implantation for impotence treatment. J Urol. 1997;157:833–39.
Queissert F, Huesch T, Kretschmer A, Anding R, Kurosch M, Kirschner-Hermanns R, et al. Artificial urinary sphincter cuff size predicts outcome in male patients treated for stress incontinence: results of a large central european multicenter cohort study. Int Neurourol J 2019;23:219–25.
Gundian JC, Barrett DM, Parulkar BG. Mayo Clinic experience with use of the AMS800 artificial urinary sphincter for urinary incontinence following radical prostatectomy. J Urol. 1989;142:1459–61.
Linder BJ, Rivera ME, Ziegelmann MJ, Elliott DS. Long-term outcomes following artificial urinary sphincter placement: an analysis of 1082 cases at Mayo Clinic. Urology. 2015;86:602–7.
Ostrowsky I, Ciechan J, Sledz E, Wojciech D, Tomasz G, Chlosta P. Four-year follow-up on a Zephyr Surgical Implants 375 artificial urinary sphincter for male incontinence from one urological center. Cent Eur J Urol. 2018;71:320–25.
Giammo A, Falcone M, Blecher G, Ammirati E, Geretto P, Manassero A, et al. A novel artificial urinary sphincter (VICTO®) for the management of postprostatectomy urinary incontinence: description of the surgical technique and preliminary results from a multicenter series. Urol Int. 2021;105:414–20.
Van der Aa F, Drake MJ, Kasyan GR, Petrolekas A, Cornu JN. The artificial urinary sphincter after a quarter of a century: a critical systematic review of its use in male non-neurogenic incontinence. Eur Urol. 2013;63:681–89.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
SW: International Medical Devices, Rigicon, Uramix. RS: Boston Scientific. JM: Boston Scientific All other authors: none. SKW wrote the article. Eric Chung provided major editing. All other authors reviewed the article and made minor editing of typos. BL electronically submitted the article. There was no funding from industry. The material presented in this manuscript contains original research, has not been previously published and has not been submitted for publication elsewhere while under consideration.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wilson, S.K., Chung, E., Langford, B. et al. First safety outcomes for rigicon conticlassic® artificial urinary sphincter. Int J Impot Res (2023). https://doi.org/10.1038/s41443-023-00748-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41443-023-00748-8