Abstract
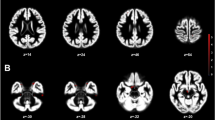
Cross-sex hormones in female-to-male (FtM) transsexuals play a crucial role in brain plasticity. Morphological study associated with white matter (WM) volume in postoperative FtM transsexuals receiving cross-sex hormones has not been published yet. This study was performed to discriminate the regional WM volume differences between postoperative FtM transsexuals and female controls using voxel-based morphometry (VBM) and further to assess the correlations between regional volume variations and cross-sex hormones. WM volume was assessed in 12 postoperative FtM transsexuals receiving cross-sex hormones with 16 age-matched female controls. WM volume was processed using SPM8 software with diffeomorphic anatomical registration via an exponentiated Lie algebra (DARTEL) algorithm. Serum sex hormones, including estriol, free testosterone (free-T), estradiol, follicle-stimulating hormone, and luteinizing hormone were measured. Postoperative FtM transsexuals showed significantly (p < 0.05) larger WM volumes in the inferior parietal lobule, postcentral gyrus, and middle temporal gyrus compared with female controls. However, there were no brain areas with larger WM volume in female controls compared with FtM transsexuals. WM volumes of the inferior parietal lobule and middle temporal gyrus in FtM transsexuals were positively correlated with the levels of free-T. This study revealed WM volume change and its correlation with free-T level in postoperative FtM transsexuals. These findings will improve our understanding of the morphometric changes in FtM transsexuals under cross-sex hormone therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 8 print issues and online access
$259.00 per year
only $32.38 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Rajkumar RP. Gender identity disorder and schizophrenia: neurodevelopmental disorders with common causal mechanisms? Schizophr Res Treat. 2014;2014:463757.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders - 5th ed. Washington DC: American Psychiatric Association 2013.
De Cuypere G, T’Sjoen G, Beerten R, Selvaggi G, De Sutter P, Hoebeke P, et al. Sexual and physical health after sex reassignment surgery. Arch Sex Behav. 2005;34:679–90.
Kim GW, Kim SK, Jeong GW. Neural activation-based sexual orientation and its correlation with free testosterone level in postoperative female-to-male transsexuals: preliminary study with 3.0-T fMRI. Surg Radiol Anat. 2016;38:245–52.
Kim TH, Kim GW, Kim SK, Jeong GW. Brain activation-based sexual orientation in female-to-male transsexuals. Int J Impot Res. 2016;28:31–38.
Flores AR, Herman JL, Gates GJ, Brown TNT. How many adults identify as transgender in the United States? Retrieved from 28 November 2016. http://williamsinstitute.law.ucla.edu/wp-content/uploads/How-Many-Adults-Identify-as-Transgender-in-the-United-States.pdf.
Fernandez-Guasti A, Kruijver FP, Fodor M, Swaab DF. Sex differences in the distribution of androgen receptors in the human hypothalamus. J Comp Neurol. 2000;425:422–35.
Kim GW, Jeong GW. Menopause-related brain activation patterns during visual sexual arousal in menopausal women: An fMRI pilot study using time-course analysis. Neuroscience. 2017;343:449–58.
Kim TH, Kim SK, Jeong GW. Cerebral gray matter volume variation in female-to-male transsexuals: a voxel-based morphometric study. Neuroreport. 2015;26:1119–25.
Zubiaurre-Elorza L, Junque C, Gomez-Gil E, Guillamon A. Effects of cross-sex hormone treatment on cortical thickness in transsexual individuals. J Sex Med. 2014;11:1248–61.
Case LK, Brang D, Landazuri R, Viswanathan P, Ramachandran VS. Altered white matter and sensory response to bodily sensation in female-to-male transgender individuals. Arch Sex Behav. 2017;46:1223–37.
Kranz GS, Hahn A, Kaufmann U, Küblböck M, Hummer A, Ganger S, et al. White matter microstructure in transsexuals and controls investigated by diffusion tensor imaging. J Neurosci. 2014;34:15466–75.
Rametti G, Carrillo B, Gomez-Gil E, Junque C, Segovia S, Gomez Á, et al. White matter microstructure in female to male transsexuals before cross-sex hormonal treatment. A diffusion tensor imaging study. J Psychiatr Res. 2011;45:199–204.
Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113.
Kim GW, Park KS, Jeong GW. Effects of sex hormones and age on brain volume in post-menopausal women. J Sex Med. 2018;15:662–70.
Kim GW, Kim YH, Jeong GW. Whole brain volume changes and its correlation with clinical symptom severity in patients with schizophrenia: a DARTEL-based VBM study. PLoS ONE. 2017;12:e0177251.
Kim SK, Moon JB, Heo J, Kwon YS, Lee KC. A new method of urethroplasty for prevention of fistula in female-to-male gender reassignment surgery. Ann Plast Surg. 2010;64:759–64.
Jeong GW, Park K, Youn G, Kang HK, Kim HJ, Seo JJ, et al. Assessment of cerebrocortical regions associated with sexual arousal in premenopausal and menopausal women by using BOLD-based functional MRI. J Sex Med. 2005;2:645–51.
Kim GW, Yoon W, Jeong GW. Whole-brain volume alteration and its correlation with anxiety severity in patients with obsessive-compulsive disorder and generalized anxiety disorder. Clin Imaging. 2018;50:164–70.
Leonard CM, Towler S, Welcome S, Halderman LK, Otto R, Eckert MA, et al. Size matters: cerebral volume influences sex differences in neuroanatomy. Cereb Cortex. 2008;18:2920–31.
Marner L, Nyengaard JR, Tang Y, Pakkenberg B. Marked loss of myelinated nerve fibers in the human brain with age. J Comp Neurol. 2003;462:144–52.
Rametti G, Carrillo B, Gomez-Gil E, Junque C, Zubiaurre-Elorza L, Segovia S, et al. Effects of androgenization on the white matter microstructure of female-to-male transsexuals. A diffusion tensor imaging study. Psychoneuroendocrinology. 2012;37:1261–9.
Bramen JE, Hranilovich JA, Dahl RE, Chen J, Rosso C, Forbes EE, et al. Sex matters during adolescence: testosterone-related cortical thickness maturation differs between boys and girls. PLoS ONE. 2012;7:e33850.
Perrin JS, Herve PY, Leonard G, Perron M, Pike GB, Pitiot A, et al. Growth of white matter in the adolescent brain: role of testosterone and androgen receptor. J Neurosci. 2008;28:9519–24.
Marszalek JR, Williamson TL, Lee MK, Xu Z, Hoffman PN, Becher MW, et al. Neurofilament subunit NF-H modulates axonal diameter by selectively slowing neurofilament transport. J Cell Biol. 1996;135:711–24.
Pol HEH, Cohen-Kettenis PT, Van Haren NEM, Peper JS, Brans RGH, Cahn W, et al. Changing your sex changes your brain: influences of testosterone and estrogen on adult human brain structure. Eur J Endocrinol. 2006;155:S107–S114.
Lessov-Schlaggar CN, Reed T, Swan GE, Krasnow RE, DeCarli C, Marcus R, et al. Association of sex steroid hormones with brain morphology and cognition in healthy elderly men. Neurology. 2005;65:1591–6.
Frederikse ME, Lu A, Aylward E, Barta P, Pearlson G. Sex differences in the inferior parietal lobule. Cereb Cortex. 1999;9:896–901.
Raznahan A, Lee Y, Stidd R, Long R, Greenstein D, Clasen L, et al. Longitudinally mapping the influence of sex and androgen signaling on the dynamics of human cortical maturation in adolescence. Proc Natl Acad Sci USA. 2010;107:16988–93.
Celec P, Ostatnikova D, Hodosy J. On the effects of testosterone on brain behavioral functions. Front Neurosci. 2015;9:12.
Guillamon A, Junque C, Gomez-Gil E. A review of the status of brain structure research in transsexualism. Arch Sex Behav. 2016;45:1615–48.
Heany SJ, van Honk J, Stein DJ, Brooks SJ. A quantitative and qualitative review of the effects of testosterone on the function and structure of the human social-emotional brain. Metab Brain Dis. 2016;31:157–67.
Liu H, Yang Y, Xia Y, Zhu W, Leak RK, Wei Z, et al. Aging of cerebral white matter. Ageing Res Rev. 2017;34:64–76.
Zatorre RJ, Fields RD, Johansen-Berg H. Plasticity in gray and white: neuroimaging changes in brain structure during learning. Nat Neurosci. 2012;15:528–36.
Butt AM, Fern RF, Matute C. Neurotransmitter signaling in white matter. Glia. 2014;62:1762–79.
Pesaresi M, Soon-Shiong R, French L, Kaplan DR, Miller FD, Paus T. Axon diameter and axonal transport: In vivo and in vitro effects of androgens. Neuroimage. 2015;115:191–201.
Kragel PA, LaBar KS. Somatosensory representations link the perception of emotional expressions and sensory experience. eNeuro. 2016;3:0090–15.
Redoute J, Stoleru S, Gregoire MC, Costes N, Cinotti L, Lavenne F, et al. Brain processing of visual sexual stimuli in human males. Hum Brain Mapp. 2000;11:162–77.
Stoleru S, Fonteille V, Cornelis C, Joyal C, Moulier V. Functional neuroimaging studies of sexual arousal and orgasm in healthy men and women: a review and meta-analysis. Neurosci Biobehav Rev. 2012;36:1481–509.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grants funded by the Korea government (MSIT) (2018R1A2B2006260 and 2018R1C1B6005456) and Chonnam National University Research Fund for CNU distinguished research emeritus professor (2017–2022).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kim, GW., Kim, YH., Park, K. et al. A comparative study of white matter volume between postoperative female-to-male transsexuals and healthy female. Int J Impot Res 31, 432–438 (2019). https://doi.org/10.1038/s41443-019-0111-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41443-019-0111-5