Abstract
Purpose
To prospectively assess patient reported outcomes and risk management behavior of women choosing to receive (receivers) or decline (decliners) their breast cancer polygenic risk score (PRS).
Methods
Women either unaffected or affected by breast cancer and from families with no identified pathogenic variant in a breast cancer risk gene were invited to receive their PRS. All participants completed a questionnaire at study enrollment. Receivers completed questionnaires at two weeks and 12 months after receiving their PRS, and decliners a second questionnaire at 12 months post study enrollment.
Results
Of the 208 participants, 165 (79%) received their PRS. Among receivers, there were no changes in anxiety or distress following testing. However, compared to women with a low PRS, those with a high PRS reported greater genetic testing–specific distress, perceived risk, decisional regret, and less genetic testing–positive response. At 12 months, breast screening and uptake of risk-reducing strategies were consistent with current Australian guidelines of breast cancer risk management. Reasons for declining PRS included being unable to attend the appointment in person and concerns over potential emotional response.
Conclusion
The outcomes of the study provide insight into women’s responses to receiving PRS and highlight the issues that need to be addressed in the associated model of genetic counseling.
Similar content being viewed by others
INTRODUCTION
Polygenic risk scores (PRS), typically calculated as the weighted sum of multiple genetic risk variants, have emerged as a potential tool for stratifying individuals into different levels of disease risk [1]. The PRS for breast cancer has been shown to improve stratification of risk in the general population [2] and to successfully identify women remaining at increased risk after exclusion of pathogenic variants in known breast cancer genes, such as BRCA1 and BRCA2 (BRCA1/2) [3,4,5]. In the familial setting, PRS can be used to inform risk management strategies for women unaffected by breast cancer, and manage the risk of contralateral disease for previously affected women [6].
Testing for breast cancer PRS is now available in clinical practice, however, important methodological and reporting elements are yet to be addressed, including the effect of ancestry on a PRS [7] and the lack of current guidelines to ensure consistency across laboratories [1, 8]. Furthermore, little is known about how this information impacts patients, as described by patient reported outcomes measures (PROMs), or influences future risk management [9]. Research to date indicates women support a personalized approach to breast cancer risk management based on polygenic information, and initial studies assessing hypothetical responses [10] or qualitative analysis [11,12,13] have documented few adverse psychological outcomes.
Prospective data on the implementation of a breast cancer PRS is needed to inform the development of effective risk communication tools and ensure provision of PRS is not associated with adverse psychological outcomes or unwanted health behaviors. This study aimed to assess short (2 weeks) and long-term (12 months) PROMs and describe risk management behavior in women receiving (receivers) or declining to receive (decliners) their personal PRS. The study examined the hypothesis that women receiving a higher PRS would have greater breast cancer–specific distress compared to those with a lower PRS in the short term, but that there would be no persistent differences in patient reported outcomes in the long term.
MATERIALS AND METHODS
Study design and setting
The study protocol, description of consultations, and uptake of the PRS have been published previously [13,14,15,16]. Women were recruited from the Variants in Practice (ViP) study, in which genotyping of 62 breast cancer–associated single-nucleotide polymorphisms (SNPs) was performed for ~3,700 women and a PRS and corresponding relative risk (RR) for breast cancer were calculated (Supplementary methods 1) [12]. Women from families with no identified pathogenic variant in a breast cancer risk gene on comprehensive testing were eligible to participate, including women who were either affected or unaffected by breast cancer. A total of 400 women meeting these criteria from either end of the PRS distribution in the ViP cohort were selected for this study, i.e., the women with the highest and lowest assessed breast cancer risk based on their PRS. Eligible women were mailed the study information and invited to receive their PRS. Of the 400 women invited, 200 had a PRS associated with an increased breast cancer risk (RR ≥ 1.21; termed PRS+) and 200 no change/reduced cancer risk (RR < 1.21; termed PRS−). The study was approved by the Human Research Ethics Committee at participating sites (HREC/16/PMCC/2 and H0016395).
Sample size and power
The final enrollment for the study was less than the projected recruitment (n = 215 receivers) [14] and did not meet the previously calculated power to investigate an interaction between the PRS and disease status. Consequently, this analysis was omitted and a post hoc power calculation was conducted. For a two-sided test based on a 5% significance level, our sample size of 153 receivers who completed the survey at 2 weeks had a greater than 90% power to detect a seven-score difference in the primary psychological outcome of breast cancer anxiety, as measured by the Impact of Event Scale (IES) (SD 14, range 0–75) between PRS+ and PRS− women. This difference is considered a medium effect size [17], as well as being clinically significant in the context of measuring psychological outcomes [18].
Disclosure of the PRS
Women received their PRS at an in-person appointment with a genetic health professional (e.g., clinical geneticists, genetic counselor, clinical oncologist) [16]. Consultations included the disclosure of, and discussion about, the implications of the PRS. A graphical representation of the participant’s PRS, including RR of breast cancer compared to the Australian general population (Supplementary Fig. 1) was provided to participants, along with a visual representation of their lifetime absolute breast cancer risk (Supplementary Fig. 1). Most clinicians also provided a verbal description of the PRS category (low, moderate, high) and the breast cancer RR based on the PRS. However, few clinicians described the risk as an absolute figure [16]. Given the research nature of the PRS, clinicians were instructed to offer risk management advice that continued to emphasize their personal and family history of breast cancer.
Data collection
Receivers completed three questionnaires: at study enrollment (baseline), two weeks after receiving their PRS (short-term), and 12 months post receipt of results (long-term) (Supplementary Fig. 2). Decliners completed the baseline survey and a questionnaire 12 months post enrollment. The study questionnaires included the IES [19], Hospital Anxiety and Depression Scale (HADS) [20], perceived risk of breast cancer [21], knowledge of familial breast cancer and PRS [22, 23], the Multidimensional Impact of Cancer Risk Assessment (MICRA) [24], and Decision Regret Scale (DRS) [25]. Likert and open-ended questions captured reasons for declining PRS (Supplementary Table 2) [21]. Self-reported data on breast screening (date of last screening and screening modality) and uptake of risk-reducing strategies (bilateral mastectomy and risk-reducing medication) were also collected [26]. Data collection occurred from August 2016 to December 2019.
Analysis of the impact of receiving PRS on PROM over time
Internal consistency was calculated using Cronbach’s alpha for each PROM (Supplementary Table 1). For analysis, receivers were stratified based on whether they received a PRS+ or PRS− result and their personal history of breast cancer (affected and unaffected).
A linear mixed model assessed impact of the PRS on PROMS over time. Interactions between time and the PRS category were considered and removed from the model if p > 0.05. For all models, a diagonal covariance structure was used, and disease status was included to account for the potential effects of personal breast cancer history. Other stressful life events that may affect psychological wellbeing [27] were also included in the analysis of the IES, HADS, and MICRA.
Uptake of risk management behaviors
Risk-reducing strategies were compared between baseline and 12-month follow-up to determine uptake during the study period. Logistic regression was used to assess the effect of the PRS on breast screening at 12 months post result. Logistic regression was conducted between predictor variables (PRS, disease status, number of first- and second-degree relatives with breast cancer, age, education level and previous attendance to genetics clinic) and the outcome of reported breast screening at 12 months (i.e., breast mammography, ultrasound, and/or magnetic resonance image [MRI]). Women’s age was dichotomized (<40 years, ≥40 years) to coincide with the age women are eligible for publicly funded mammographic screening in Australia, in the setting of family history of breast cancer [28]. Variables where p < 0.25 in univariate analyses were included in the multivariate model. A backwards elimination strategy was employed where p < 0.05 was considered statistically significant.
Analysis of decliners PROMs and comparison to receivers
Mean differences in IES and HADS scores between receivers and decliners at 12 months were assessed using an analysis of covariance (ANCOVA), adjusted for IES and HADS scores measured at baseline. An independent sample t-test was used to assess mean differences in decisional regret between receivers and decliners. Descriptive statistics were used to describe the frequency of agreement for reasons to not receive PRS, and content analysis was used to evaluate responses to open-ended question regarding additional reasons to not receive their PRS. All statistical analyses were performed using SPSS version 25 [29].
RESULTS
Participant characteristics
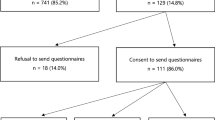
Of the 400 women invited to the study, 208 (52.0%) consented to participate and completed the baseline questionnaire, 184 (46.0%) did not participate, and 8 (2.0%) were deceased [15]. Of the participants, 165 (79.3%) received their personal PRS and 43 (20.7%) declined (Supplementary Fig. 2) [15]. The mean age of the cohort at baseline was 52.3 years (SD = 13.1). Most women had at least one daughter (57.8%), completed a bachelor’s degree (53.1%), and were born in Australia (87.4%) (Table 1). For affected women, the mean age of their first breast cancer diagnosis was 45.1 years (SD 11.5, range 24 to 75 years). Among receivers, 104 (63%) were categorized as PRS+ and 61 (37.0%) as PRS−. The mean breast cancer RR generated from the PRS was 0.7 (SD 0.2, range 0.2 to 1.1) for PRS− women, and 2.2 (SD 0.6, range 1.2 to 3.8) for PRS+. Stratified by personal history, the mean breast cancer RR was 1.8 (SD 1.0) for affected women and 1.4 (SD 0.7) for unaffected women (Supplementary Fig. 3). One woman had a personal history of ovarian cancer, and 22 had a family history on ovarian cancer in a first or second-degree relative.
Retention rate at 12 months was 60.5% for decliners and 87.3% for receivers (Supplementary Fig. 2). There were no significant differences in age, education level, disease status, perceived risk, knowledge of familial breast cancer, general depression and anxiety (HADS), or breast cancer–specific distress (IES) at baseline between participants who were retained and those lost to follow-up. However, receivers were significantly more likely to complete the study compared to decliners, X2(2, n = 208) = 17.5, p < 0.001.
Recall of the PRS received
Recall of the verbal description of the PRS category was high. At 12 months, most women (90.8%, n = 129) correctly recalled the verbal description of their PRS category (high or low). Four women could not recall their PRS category, and five incorrectly reported they received a low PRS when they had received a high PRS (all had a breast cancer RR > 2). Finally, three women who received a high PRS responded that they did not receive a definite result.
Impact of receiving PRS on PROMS over time
Breast cancer–specific distress and anxiety (IES) and general anxiety and depression (HADS)
At baseline, the mean IES score for all receivers was 9.5 (SD 13.9), of a total of 75, indicating mild distress and anxiety related to breast cancer risk (Table 2) [19]. Similarly, there was mild general anxiety and depression in this population (M 7.8, out of 21, SD 5.5) (Table 2) [20]. Over the course of the study, individual IES and HADS scores did not change and the PRS result received had no effect (Table 3). However, affected women reported an IES score an average of 1.3 units higher (p < 0.001) than unaffected women at all time points; mean score at baseline 13.4 (SD 15.4) and 5.9 (SD 11.0), respectively. Affected women also reported a HADS score on average 2.3 units higher than unaffected women at all time points (p < 0.001); mean score at baseline 8.4 (SD 6.1) and 7.1 (SD 4.5), respectively. Those who reported more stressful life events at 12 months also reported significantly higher IES (p = 0.004) and HADS scores (p < 0.001).
Breast cancer perceived risk
The mean breast cancer perceived risk score at baseline was 51.7 (SD 24.0), on a scale of 0 (no chance) to 100 (will definitely develop breast cancer) (Table 2). Compared to baseline, there was a significant decrease in perceived risk at two weeks (p = 0.023) and 12 months (p = 0.030) (Table 3). The size of this effect was dependent on the PRS received, with PRS− women reporting significantly lower perceived risk at 12 months (M 41.0, SD 23.9) when compared to PRS+ women (M 54.8; SD 25.2) (p = 0.023). Affected women also reported a higher score than unaffected women, mean baseline scores 54.1 (SD 24.7) and 49.3 (SD 23.1), respectively (p = 0.022).
Knowledge of familial breast cancer and PRS
At baseline, the mean knowledge score was 6.8 (out of 10, SD 1.8). Compared to baseline, knowledge scores were on average 0.5 units higher at two weeks, representing a very small, but significant increase in familial breast cancer knowledge (p = 0.023) (Table 3). This effect was not dependent on the PRS received or affected status. Despite this increase, further evaluation of individual items identified that knowledge of specific elements remained low for all receivers (Supplementary Fig. 4). At baseline, only 7.3% of receivers correctly identified that the breast cancer PRS was not associated with increased ovarian cancer risk. At 12 months, 13.5% of participants were able to correctly answer this question, indicating that most women continued to believe the breast cancer PRS was associated with ovarian cancer risk. Among those with a family history of ovarian cancer (n = 22), 10 completed the 2-week survey, of whom 5 correctly answered that their breast cancer PRS could not be used to inform ovarian cancer risk. This knowledge remained consistent at 12 months for women in this group.
Genetic testing–specific response (MICRA)
At two weeks, the mean genetic testing–specific distress was 3.4 (out of 30, SD 5.0). Distress was on average 1.6 units greater in PRS+ women compared to PRS− (p = 0.004) (Table 3). Similarly, distress in affected women was on average 1.8 units higher than unaffected; mean score at two weeks 4.5 (SD 4.8) and 2.3 (SD 5.0), respectively.
The mean genetic testing–specific uncertainty at two weeks was 7.2 (out of 45, SD 6.9) (Table 2). The PRS result had no effect on this outcome, but uncertainty was on average 3.6 units higher in affected women than unaffected (p < 0.001); mean 9.5 (SD 6.6) and 4.8 (SD 6.3), respectively. Higher distress and uncertainty were also reported by those with greater stressful life event score at 12 months (distress: p = 0.023, uncertainty: p = <0.001). Over the course of the study, individual distress or uncertainty scores did not change, with the differences identified sustained at 12 months.
At two weeks, the mean positive experience score was 9.7 (out of 20, SD 5.4), indicating some positive response to receiving the PRS (Table 2). Positive response was on average 4.3 units lower in PRS+ women compared to PRS− (p < 0.001). Between 2 weeks and 12 months, there was a 4.1 unit decrease in positive response (p < 0.001) (Table 3). This change was not dependent on the PRS received, indicating both PRS+ and PRS− women reported lower positive experiences by 12 months. Personal history of breast cancer and stressful life events did not significantly affect this outcome.
Decisional regret over receiving PRS
Most receivers (57.4%) reported no regret (score = 0/100) regarding the decision to receive their PRS at 2 weeks and these scores did not change over the course of the study (Table 3). On average PRS+ women reported a score 3.4 units higher than PRS− women (p = 0.031). However, these scores were still within the range of minimal regret (Table 2). There was no effect of personal history of breast cancer on this measure.
Risk management behavior
Risk-reducing strategies (n = 208)
In this cohort of women from breast cancer families, risk-reducing measures were common at baseline: 35 women reported having a bilateral mastectomy, including one unaffected individual (Table 4). Of the four women who had undergone bilateral mastectomy at 12 months, three were receivers and one decliner, and all had a personal history of breast cancer with at least one of the following features: young age of diagnosis (<40 years), bilateral disease, and increased risk PRS. Of the 43 women taking tamoxifen at baseline, 41 (95.3%) were taking it as adjuvant therapy and two unaffected women as prevention (4.7%). At 12 months, six women began taking risk-reducing medication and uptake of these strategies was limited to affected women (Table 4).
Breast screening (n = 158)
Based on the date of their last reported breast screen, women who had not undergone a bilateral mastectomy at baseline were categorized as never screened (n = 13, 8.2%), distant (last screening >5 years ago; n = 17, 10.8%), somewhat distant (3 to 4 years ago; n = 2, 1.3%), and recent screening (<2 years; n = 126, 79.7%). Among women who reported recent screening, most had mammograms (n = 113, 89.7%) and/or breast ultrasounds (n = 85, 67.4%). Among the 27 women who had a recent breast MRI, 17 (63.0%) were aged 49 years or younger. The 13 participants who reported never having any breast screening were all unaffected and aged between 24 and 42 years.
At 12 months, 139 (81.7%) women provided information regarding their breast screening in the preceding year since receiving their PRS (receivers) or enrollment (decliners). Of these most had undertaken mammograms (n = 96, 93.2%) and/or breast ultrasounds (n = 59, 57.2%), or MRIs (n = 14, 8.3%). Eight women who had never been screened (n = 1), reported distant (n = 6), or somewhat distant (n = 1) screening undertook screening within the study period. Of these eight women, five were PRS+, two PRS−, and one decliner.
Multivariable logistic regression indicated that affected women (odds ratio [OR] = 4.9, p = 0.025), over the age of 40 years (OR = 8.2, p < 0.001), and those who had attended a familial cancer clinic prior to study enrollment (OR = 3.1, p = 0.03) were more likely to report having breast screening at 12 months (Table 5). There was no effect of the PRS, level of education, or number of relatives diagnosed with breast cancer on breast screening at 12 months. Overall, no evidence was found for disproportionate or clinically unjustified decisions relating to uptake of risk management strategies in the 12 months after receiving a personal PRS result.
Comparison between decliners and receivers
There were no significant differences in IES (p = 0.07) and HADS scores (p = 0.86), adjusted for baseline values between decliners and receivers at 12 months. However, decliners reported a significantly higher regret over their decision to not receive their PRS (M 37.9, SD 16.2) when compared to receivers (M 9.3; SD 15.1) (p < 0.001). Decliners most frequently rated the reason for not receiving their PRS as they were “happy with their lives right now” (72.0%), because they felt they were “already aware of their level of breast cancer risk” (41.7%) and “the test will not tell me when I will develop breast cancer” (36.0%) (Supplementary Table 2). In the open-ended responses, decliners also described other concerns such as being unable to attend appointment in person, already undertaking appropriate breast cancer risk management and concerns over emotional response (Supplementary Table 3).
DISCUSSION
Several studies have demonstrated the potential for improved clinical outcomes from the use of a PRS for breast cancer risk [1, 3,4,5], however, few have described patient responses to receiving this information. Our study provides the detailed prospective examination of PROMs and risk management behavior in the year following receipt of a PRS. The findings indicate that women report minimal adverse psychological impact up to 12 months post receipt of result. Receiving a PRS was associated with adjustments in perceived breast cancer risk, which were sustained at 12 months. Persistent knowledge gaps were also identified, highlighting a need for additional educational resources to support the communication of PRS in clinical practice. Lived experiences, including personal history of breast cancer and the presence of stressful life events, proved to be strong predictors of psychological wellbeing. This finding is in line with research evaluating outcomes of BRCA1/2 testing, which consistently reported increased distress among women with a personal history of breast cancer [30]. Together these data provide important insights into the requirements for a successful practice model that incorporates the breast cancer PRS. Such a model would need to consider the personalized nature of the PRS, and how it differs from monogenic information, while also continuing to acknowledge the importance of patients’ lived experiences as a major influence on how they cope and adjust to genetic risk information.
Although it was not possible to evaluate the accuracy of perceived risk in this population, it is evident that women overestimated their level of risk (mean self-estimated lifetime risk at baseline 52%). Despite adjustments in risk perception among PRS− women at 12 months, the mean estimated absolute risk in this population remained inflated (mean 41%). This finding is consistent with previous reports that women overestimate their absolute risk of breast cancer and generally have poor numerical recall [31]. Instead women are more likely to perceive their level of risk categorically, with verbal descriptions such as “high” or “probable” [32]. In contrast to numerical estimates, women’s self-categorization has also been shown to be largely consistent with estimates provided by health professionals [32, 33]. Other factors widely reported as influencing perceived risk include lived experiences related to cancer and emotional state to cancer [31, 32].
Based on the psychometric properties of the measures used, women in our cohort reported minimal adverse psychological outcomes post receipt of the PRS. However, compared to those with a low PRS, women with a high PRS reported greater genetic testing–specific distress, perceived risk, decisional regret and less genetic testing–positive response. These findings are in line with previous studies evaluating women’s responses to BRCA1/2 genetic testing, with women who are heterozygous for a pathogenic/likely pathogenic variant in these genes more likely to report negative psychological outcomes, compared women without a variant. Thus, as with other forms of genetic testing, our findings highlight that attention needs to be paid to psychological outcomes associated with receiving PRS, including that woman at higher genetic risk may require additional support following receipt of result.
In the first year following PRS testing the additional uptake of risk-reducing strategies in this group was low. Most women received a PRS that equated to a moderately increased risk of breast cancer (Supplementary Fig. 3) [34], and in the absence of a strong family history, this level of risk would rarely be sufficient to prompt a recommendation for risk-reducing surgery. Based on this risk, the uptake of risk-reducing bilateral mastectomy was in line with current national guidelines of breast cancer risk management. Uptake of risk-reducing salpingo oophorectomy (RRSO) was not evaluated in our study.
At baseline, most women reported regular breast screening, consistent with evidence that women continued to engage in screening despite previously receiving negative BRCA1/2 genetic testing results [35]. To date, there is limited evidence of the impact of PRS on cancer risk management, and concerns have been raised over the potential for negative health behaviors among those receiving a low PRS [9]. Our findings indicate that although PRS− women reported significantly lower perceived risk, the PRS result was not an independent predictor of breast screening, reflecting the fact that the PRS− group of women continued to engage in screening. Previous qualitative evidence suggests that women are able to place their PRS in the context of multifactorial nature of breast cancer risk, including family history and lifestyle factors [13]. Additionally, women who receive a high PRS result reported greater awareness of their breast cancer risk and felt empowered to access appropriate risk management strategies [13]. Future studies should aim to further explore the impact of receiving PRS on motivation to have, and long-term adherence to, breast screening in a larger cohort. It is also important to highlight that there are no clinical guidelines on the use of breast cancer PRS and there is limited data on the extent in which the PRS improves clinical outcomes [1]. To address this limitation, several clinical trials of breast cancer PRS are now underway in Australia (e.g., PRiMo [36]) and internationally (e.g., WISDOM [37]). These trials will generate important data on the clinical utility of breast cancer PRS and provide a framework for the implementation of this test in clinical practice.
Very little is known about individuals who decline to receive genetic information. We identified that decliners experienced significantly greater decisional regret. Decliners also report fewer perceived benefits and greater practical and emotional concerns about receiving their PRS when compared to receivers [15]. Similar concerns have been reported across other populations including individuals notified about the availability of genetic testing for melanoma [21] and ovarian cancer risk [38]. These findings emphasize the need to continue to improve access to genetic health services. Genetic counseling for familial breast cancer is ideally placed for widespread implementation of telehealth services, with studies reporting this model to be cost effective [39] and comparable to in-person consultations. The decisional regret reported among decliners also suggests some women may require additional support to facilitate genetic testing decisions. Tools such as decision aids can assist individuals making decisions about whether to take up genetic counseling and testing [40].
This study provides insight into the impact of a PRS-based breast cancer risk assessment on PROMs and risk management behavior over a significant period of follow-up. The study found that PRS results were acceptable with no evidence for clinically significant adverse psychological outcomes or negative effects on health behavior. However, these findings should be interpreted in light of the study limitations. There was little diversity in this cohort demographically with nearly all women born in Australia and speaking English at home. Thus, generalizability to other cultural and linguistic groups is limited. Consideration also needs to be given to the low retention rate among decliners and findings for this group of women should be interpreted with caution.
Women were also recruited from families that had previously tested negative for pathogenic variants in monogenic risk genes. Similarly, it is likely that women self-selected to participate in the study based on interest in receiving their PRS (uptake of PRS laid between 62% and 42%) [15]. As previously reported, this cohort is comprised of early adopters [15], with women who elected to receive their PRS being more likely to have completed higher level education, and reported greater benefits and fewer barriers and concerns about receiving their results than decliners. It is possible that these differences are reflected in the reported PROMs, which may not be fully representative of all women offered polygenic testing. Women also received their PRS from a genetic health professional and the findings may not be representative of women who receive PRS from other health providers and do not receive genetic counseling. Finally, with a relatively small cohort, the study was not powered to evaluate impact on risk management behavior, and findings were limited to 12-month follow-up. Nevertheless, the outcomes of the study provide insight into women’s responses to receiving PRS and highlight the issues that need to be addressed in the associated model of genetic counseling. Future studies are warranted to assess clinical benefits of providing PRS including long-term adherence to breast screening, effect on health outcomes, and cost–benefit analysis.
Data availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
Yanes T, Young M-A, Meiser B, James PA. Clinical applications of polygenic breast cancer risk: a critical review and perspectives of an emerging field. Breast Cancer Res. 2020;22:21.
Mavaddat N, et al. Polygenic risk scores for prediction of breast cancer and breast cancer subtypes. Am J Hum Genet. 2019;104:21–34.
Sawyer S, et al. A role for common genomic variants in the assessment of familial breast cancer. J Clin Oncol. 2012;30:4330–4336.
Lakeman IMM, et al. Addition of a 161-SNP polygenic risk score to family history-based risk prediction: impact on clinical management in non-BRCA1/2 breast cancer families. J Med Genet. 2019;56:581–589.
Evans DG, et al. The impact of a panel of 18 SNPs on breast cancer risk in women attending a UK familial screening clinic: a case-control study. J Med Genet. 2017;54:111–113.
Robson ME, et al. Association of common genetic variants with contralateral breast cancer risk in the WECARE Study. J Natl Cancer Inst. 2017;109:djx051.
Ho W-K, et al. European polygenic risk score for prediction of breast cancer shows similar performance in Asian women. Nature Commun. 2020;11:3833.
Yanes T, McInerney-Leo AM, Law M, Cummings S. The emerging field of polygenic risk scores and perspective for use in clinical care. Hum Mol Genet. 2020;29:R165–R176.
Yanes T, Willis AM, Meiser B, Tucker KM, Best M. Psychosocial and behavioral outcomes of genomic testing in cancer: a systematic review. Eur J Hum Genet. 2019;27:28–35.
Henneman L, Timmermans DR, Bouwman CM, Cornel MC, Meijers-Heijboer H. ‘A low risk is still a risk’: exploring women’s attitudes towards genetic testing for breast cancer susceptibility in order to target disease prevention. Public Health Genomics. 2011;14:238–247.
Young MA, et al. Making sense of SNPs: women’s understanding and experiences of receiving a personalized profile of their breast cancer risks. J Genet Couns. 2018;27:702–708.
Forrest LE, Sawyer SD, Hallowell N, James PA, Young M-A. High-risk women’s risk perception after receiving personalized polygenic breast cancer risk information. Journal of Community Genetics. 2019;10:197–206.
Yanes T, et al. Women’s responses and understanding of polygenic breast cancer risk information. Fam Cancer. 2020;19:297–306.
Yanes T, et al. Psychosocial and behavioral impact of breast cancer risk assessed by testing for common risk variants: protocol of a prospective study. BMC Cancer. 2017;17:491.
Yanes T, et al. Uptake of polygenic risk information among women at increased risk of breast cancer. Clin Genet. 2020;97:492–501.
Das Gupta et al. Communicating polygenic risk scores in the familial breast cancer clinic. Patient Educ Couns. 2021;S0738-3991:00168-3.
Cohen J. Statistical power analysis for the behavioural sciences. 2nd ed. New York: Academic Press; 1988.
Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003;41:582–592.
Horowitz M, Wilner N, Alvarez W. Impact of Events Scale: a measure of subjective stress. Psychosomatic Med. 1979;41:209–218.
Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67:361–370.
Kasparian NA, Meiser B, Butow PN, Simpson JM, Mann GJ. Genetic testing for melanoma risk: a prospective cohort study of uptake and outcomes among Australian families. Genet Med. 2009;11:265–278.
Erblich J, Brown K, Kim Y, Valdimarsdottir HB, Livingston BE, Bovbjerg DH. Development and validation of a Breast Cancer Genetic Counseling Knowledge Questionnaire. Patient Educ Couns. 2005;56:182–191.
Ondrusek N, Warner E, Goel V. Development of a knowledge scale about breast cancer and heredity (BCHK). Breast Cancer Res Treat. 1999;53:69–75.
Cella D, et al. A brief assessment of concerns associated with genetic testing for cancer: The multidimensional impact of cancer risk assessment (MICRA) questionnaire. Health Psychol. 2002;21:564–572.
Brehaut JC, et al. Validation of a decision regret scale. Med Decis Making. 2003;23:281–292.
Price MA, et al. Predictors of breast cancer screening behavior in women with a strong family history of the disease. Breast Cancer Res Treat. 2010;124:509–519.
Brugha T, Cragg D. The List of Threatening Experiences: the reliability and validity of a brief life events a a questionnaire. Acta Psychiatr Scand. 1990;82:77–81.
Breast Screen Australia. BreastScreen Australia National Policy: eligibility to access BreastScreen Australia Services. 2018. http://www.cancerscreening.gov.au/internet/screening/publishing.nsf/Content/br-policy-for-BreastScreen-Australia-Eligibility.
SPSS Statistics for Windows [computer program]. Version 25.0. Armonk, NY. Released 2017.
Beran TM, et al. The trajectory of psychological impact in BRCA1/2 genetic testing: does time heal? Ann Behav Med. 2008;36:107–116.
Sivell S, et al. How risk is perceived, constructed and interpreted by clients in clinical genetics, and the effects on decision making: systematic review. J Genet Couns. 2007;17:30–63.
Fielden HG, Brown SL, Saini P, Beesley H, Salmon P. How do women at increased breast cancer risk perceive and decide between risks of cancer and risk-reducing treatments? A synthesis of qualitative research. Psychooncology. 2017;26:1254–1262.
Katapodi MC, Lee KA, Facione NC, Dodd MJ. Predictors of perceived breast cancer risk and the relation between perceived risk and breast cancer screening: a meta-analytic review. Prev Med. 2004;38:388–402.
EviQ Cancer Treatments. Risk Management for Unaffected Women at Moderately Increased Risk of Breast Cancer. 2021; https://www.eviq.org.au/Category/tabid/65/categoryid/66/Default.aspx.
van Dijk S, et al. What’s the message? Interpretation of an uninformative BRCA1/2 test result for women at risk of familial breast cancer. Genet Med. 2005;7:239–245.
Australian New Zealand Clinical Trials Registry. Effect of Polygenic Risk Modification on breast cancer risk management and prevention: The PRiMo Trial (ACTRN12621000009819). 2020. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=380649&showOriginal=true&isReview=true.
Esserman LJ, WISDOM Study and Athena I. The WISDOM Study: breaking the deadlock in the breast cancer screening debate. npj Breast Cancer. 2017;3:34.
Smit AK, et al. Distress, uncertainty, and positive experiences associated with receiving information on personal genomic risk of melanoma. Eur J Hum Genet. 2018;26:1094–1100.
Buchanan AH, et al. Randomized trial of telegenetics vs. in-person cancer genetic counseling: cost, patient satisfaction and attendance. J Genet Couns. 2015;24:961–970.
Willis AM, et al. Development and pilot testing of a decision aid for genomic research participants notified of clinically actionable research findings for cancer risk. J Genet Couns. 2018;27:1055–1066.
Acknowledgements
We thank all the women who participated in the study as well as all the clinicians at the participating familial cancer clinics for accommodating this study. This study is supported by a grant from the Cancer Council of New South Wales (ID: 1079897). T.Y. was supported by a National Health and Medical Research Council (NHMRC) and National Breast Cancer Foundation postgraduate scholarship (ID: 1133049), and a Translational Cancer Research Institute PhD Top-up Scholarship. B.M. was supported by an NHMRC Senior Research Fellowship Level B (ID 1078523).
Author information
Authors and Affiliations
Contributions
Conceptualization: T.Y., B.M., M.A.Y., K.B.S., Y.A., P.J. Data curation: T.Y., B.M., R.K., M.S.J., S.M. Formal Analysis: T.Y., B.M., R.K., B.B.S., P.J. Funding acquisition: TY, B.M., M.A.Y., K.B.S., Y.A., P.J. Investigation: T.Y., B.M., R.K., M.A.Y., P.B.M., M.S.J., S.M., S.T., K.B.S., Y.A., L.S., C.S., B.B.S., P.A.J. Methodology: T.Y., B.M., M.A.Y., K.B.S., Y.A., P.J. Project administration: B.M., M.A.Y., K.B.S. P.J. Supervision: B.M., M.A.Y., K.B.S. P.J. Validation: B.M., M.A.Y., K.B.S. P.J. Visualization: T.Y., B.M., M.A.Y., K.B.S. P.J. Writing—original draft: T.Y., B.M. Writing—review & editing: T.Y., B.M., R.K., M.A.Y., P.B.M., M.S.J., S.M., S.T., K.B.S., Y.A., L.S., C.S., B.B.S., P.A.J.
Corresponding author
Ethics declarations
Ethics Declaration
The study was approved by the Human Research Ethics Committee at participating sites (HREC/16/PMCC/2 and H0016395). Informed consent was obtained for each enrolled study participant.
Competing interests
B.M. has a remunerated consultant role with the company AstraZeneca with respect to an unrelated project. AstraZeneca has not been involved in the collection or analysis of data for articles nor in writing or submitting the manuscript. The other authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
About this article
Cite this article
Yanes, T., Meiser, B., Kaur, R. et al. Breast cancer polygenic risk scores: a 12-month prospective study of patient reported outcomes and risk management behavior. Genet Med 23, 2316–2323 (2021). https://doi.org/10.1038/s41436-021-01288-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41436-021-01288-6
This article is cited by
-
Polygenic Risk Scores for Breast Cancer
Current Breast Cancer Reports (2024)
-
A qualitative study exploring the consumer experience of receiving self-initiated polygenic risk scores from a third-party website
European Journal of Human Genetics (2023)
-
The role of polygenic risk scores in breast cancer risk perception and decision-making
Journal of Community Genetics (2023)
-
Clinical utility of polygenic risk scores: a critical 2023 appraisal
Journal of Community Genetics (2023)
-
Oncobiology and treatment of breast cancer in young women
Cancer and Metastasis Reviews (2022)