Abstract
Purpose
To elucidate the novel molecular cause in families with a new autosomal recessive neurodevelopmental disorder.
Methods
A combination of exome sequencing and gene matching tools was used to identify pathogenic variants in 17 individuals. Quantitative reverse transcription polymerase chain reaction (RT-qPCR) and subcellular localization studies were used to characterize gene expression profile and localization.
Results
Biallelic variants in the TMEM222 gene were identified in 17 individuals from nine unrelated families, presenting with intellectual disability and variable other features, such as aggressive behavior, shy character, body tremors, decreased muscle mass in the lower extremities, and mild hypotonia. We found relatively high TMEM222 expression levels in the human brain, especially in the parietal and occipital cortex. Additionally, subcellular localization analysis in human neurons derived from induced pluripotent stem cells (iPSCs) revealed that TMEM222 localizes to early endosomes in the synapses of mature iPSC-derived neurons.
Conclusion
Our findings support a role for TMEM222 in brain development and function and adds variants in the gene TMEM222 as a novel underlying cause of an autosomal recessive neurodevelopmental disorder.
Similar content being viewed by others
INTRODUCTION
Intellectual disability (ID) is a clinically and genetically heterogeneous group of developmental disorders, characterized by significant limitations in both intellectual functioning and adaptive behavior1. ID has an estimated prevalence of approximately 1–3% in the general population, although a higher prevalence is observed in less-developed countries and in populations with a high frequency of consanguineous marriages2,3,4. Considering that 30–50% of the known human protein coding genes are expressed in the brain5,6, and calculations based on the 125 known X-linked ID genes and the 821 genes in the X chromosome7,8,9, it is estimated that variants in the range of 2,000–3,000 genes may be involved in the genetic basis of Mendelian ID, of which half have not yet been identified8,10,11. To date, more than 1,300 ID genes are listed in the SysID database (https://sysid.cmbi.umcn.nl)12. It has been estimated that most of the unknown gene–disease associations are caused by pathogenic variants in genes resulting in autosomal recessive modes of inheritance13,14. The discovery of genetic autosomal recessive causes of ID has been facilitated by the study of consanguineous families because of large genomic region in homozygosity15.
TMEMs are a heterogeneous group of transmembrane proteins that span the lipid bilayer of various cell membranes, such as lysosomes, mitochondrial membranes, Golgi apparatus, and endoplasmic reticulum16,17. A variety of different biological functions have been assigned to TMEM proteins, such as neurite modulation (SLITRK2)18, transmembrane calcium influx (ORAI1)19, protein glycosylation (TMEM165)20, and regulation of lysosomal trafficking (TMEM106B)21. However, the function of the majority of TMEM proteins remains unclear. This is mainly due to difficulties in extraction and purification of these transmembrane proteins. Pathogenic biallelic variants in TMEM genes have been found in patients with autosomal recessive neurodevelopmental disorders, for example Joubert syndrome 6 (OMIM 610688, TMEM67), Joubert syndrome 14 (OMIM 614424, TMEM237), Meckel syndrome 2 (OMIM 603194, TMEM216), syndromic ID (OMIM 618316, TMEM94), and muscular dystrophy–dystroglycanopathy (OMIM 615041, RXYLT1). Transmembrane protein 222, encoded by TMEM222 at 1p36.11, is a 208–amino acid protein of unknown function, comprising three transmembrane (TM) domains and a domain of unknown function (DUF778)22.
Here, we report nine unrelated families with a total of 17 affected individuals, presenting with autosomal recessive ID and variable associated phenotypes using exome sequencing and a gene matchmaking approach23, with deleterious variants in TMEM222 (NM_032125.2) as the likely cause of this syndrome.
MATERIALS AND METHODS
Family recruitment
Individuals were identified in different centers worldwide in diagnostic or research settings (Table 1). TMEM222 variants were identified by exome sequencing (ES) and confirmed by Sanger sequencing (Supplemental data, Table 1).
Gene expression profile
For TMEM222 expression analysis, total RNA from 13 brain areas, 9 fetal tissues, and 10 adult tissues was obtained from Stratagene (La Jolla, CA, USA), BioChain (Newark, CA, USA), and Clontech (Mountain View, CA, USA) (Fig. 2). The concentration of the extracted total RNA was estimated using a NanoDrop spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA). For quantitative reverse transcription polymerase chain reaction (RT-PCR), 2 µg total RNA was reverse transcribed into complementary DNA (cDNA) using the SuperScript VILO Master Mix (catalog number 11755050, Thermo Fisher Scientific, Waltham, MA, USA), following manufacturer’s instructions. Amplifications were performed in duplicates of 25 μl reactions in the presence of SYBR green (Applied Biosystems, Foster City, CA, USA), using the absolute quantification setting on an ABI PRISM 7900HT Sequence Detection System (Applied Biosystems), using the ΔΔCt method to quantify differences in TMEM222 gene expression compared with housekeeping genes GUSB, PPIB, and CLK224, as described previously25. Primer sequences are provided upon request.
TMEM222 expression vector
To amplify the genomic region encompassing the coding sequence of the TMEM222 gene, we designed PCR primers using Clontech infusion primers program selecting EcoR1 as digestion enzyme. The PCR reaction mixtures (50 μl) contained 25 μM of each primer pair, a high-fidelity (HF) Taq polymerase Phusion® High-Fidelity DNA Polymerase (catalog number M0530L; New England BioLabs, Ipswich, MA, USA), 1× PCR HF buffer, 10 mM of dNTP mix, 1× Q solution (catalog number 1005485, Qiagen, Hilden, Germany), and 5 ng cDNA library of small intestine by Clontech. Amplifications were performed using a two-phase touchdown PCR program described previously. The resulting PCR products were run on a 0.8% agarose gel and purified with the NucleoSpin Gel and PCR cleanup kit (catalog number 740609.250; Macherey-Nagel, Düren, Germany), according to the manufacturer’s protocol. Then, 150 ng of each purified insert was cloned into the linearized C2-GFP vector by using infusion Enzyme Mix (catalog number 011614; Clontech). The C2-GFP-TMEM222 cloned construct was validated by Sanger sequencing. Additionally, 150 ng of the purified insert was cloned into the pDONR201 vector by using the Gateway BP Clonase Enzyme Mix (catalog number 11789021; Thermo Fisher Scientific). The different pDONR cloned constructs were validated by Sanger sequencing, and 150 ng was cloned into the destination vector pcDNA3native/DEST by using the Gateway LR Clonase enzyme mix (catalog number 11791043; Thermo Fisher Scientific). Primer sequences are provided upon request.
HEK293T cells were transfected with wild-type TMEM222 expression gateway construct with FuGene HD Transfection Reagent (Promega, Madison, WI, USA). Transfected and control lines were lysed using standard lysis buffer with protease inhibitor cocktail (catalog number 11697498001, Roche Diagnostics, Rotkreuz, Switzerland). Proteins were separated using standard PAGE separation, transferred to a nitrocellulose membrane and detected by western blotting. The following antibodies were used: antitubulin (dilution 1∶2,000; catalog number T5326, Sigma Aldrich, St. Louis, MO, USA) and anti-TMEM222 (dilution 1∶2,000; catalog number NBP2-49295, Novus Biologicals, Centennial, CO, USA), and secondary antibodies antirabbit IRDye 800 (catalog number 9263221, LI-COR Biosciences, Bad Homburg vor der Höhe, Germany) and antimouse HRP (catalog number 115035062, Jackson ImmunoResearch Laboratories, Ely, UK) diluted 1:10,000.
Cell lines and culture maintenance
HEK293T cells (ATCC CRL-3216; American Type Culture Collection [ATCC], Wesel, Germany) were grown in DMEM supplemented with 10% (volume/volume) fetal calf serum (catalog number F7524; Sigma Aldrich), 1% (volume/volume) Na pyruvate (catalog number S8636; Sigma Aldrich), and penicillin/streptomycin (catalog number P4333; Sigma Aldrich).
Human induced pluripotent stem cells (iPSCs) 409B2 were obtained from Riken BRC—Cell engineering division (cell number HPS0076:409B2, Koyadai, Japan). Cells were maintained in E8 Flex media (STEMCELL Technologies, Vancouver, Canada) in 5% CO2. Medium was changed every two days and at appropriate confluency; cells were split 1:8 or 1:10 using ReLeSR (STEMCELL Technologies, Vancouver, Canada), an enzyme-free reagent for dissociation.
Induced pluripotent stem cell differentiation into excitatory neurons (iNeurons)
We used a rapid and reliable protocol to produce hiPSC-derived cortical glutamatergic neurons as previously described26. In brief, for the generation of rtTA/Ngn2-positive hiPSCs, two lentiviral vectors were used to stably integrate the rtTA and Ngn2 transgenes into the genome of the hiPSCs using the transfer vectors pLVX-EF1α-(Tet-On-Advanced)-IRES-G418(R) and pLVX-(TRE-thight)-(MOUSE)Ngn2-PGK-Puromycin(R), respectively. Selection of rtTA/Ngn2-positive colonies was performed with puromycin and G418. The differentiation of rtTA/Ngn2-positive hiPSCs to neurons overexpressing the transcription factor neurogenin-2 is carried out for four weeks in coculture with rat astrocytes, where the hiPSC-derived neurons show a neuronal-like morphology.
Subcellular localization
iNeurons were cultured on glass coverslips in a 24-well plate. After washing with cold phosphate-buffered saline (PBS) buffer, cells were fixed with 2 mL 4% paraformaldehyde for 20 minutes. Cells were then washed three times with cold PBS and permeabilized with 2 mL PBS with 0.1% Triton X-100 for 15 minutes and washed four times with cold PBS. For the colocalization experiments, the coverslips were incubated in anti-TMEM222 polyclonal antibody (catalog number NBP2-49295; Novus Biologicals) diluted 1:500 in 2% BSA with: anti-GM130 (catalog number 610822, BD Biosciences), anti-PSD95 (MA1-045, Thermo Fisher Scientific), anti-Synapsin 1 (catalog number 106001; Synaptic Systems, Göttingen, Germany), anti-MAP2 (catalog number 188004; Synaptic Systems), anti-EEA1 (catalog number ab70521, Abcam, Cambridge, UK), anti-Rab5 (catalog number ab66746, Abcam), anti-KIF16B (catalog number SC-390309, Santa Cruz Biotechnology, Dallas, TX, USA), anti-Sec31A (catalog number 6036944, BD Biosciences), anti-SAR1A (catalog number ab77029, Abcam), anti-NKHC1 (catalog number SC-398759, Santa Cruz Biotechnology) and anti-LMAN1 (catalog number NBP2-03381, Novus Biologicals) diluted 1:500 in 2% bovine serum albumin (BSA). Coverslips were incubated for 3 hours at room temperature and washed three times with PBS and then incubated with the secondary antibodies, goat antirabbit Alexa Fluor 488 (catalog number A11008, Life Technologies, Carlsbad, CA, USA), goat antimouse 568 (catalog number A11031, Life Technologies), and goat anti–guinea pig (catalog number A21450, Life Technologies) diluted 1:1,000 for 1 hour at room temperature. The coverslips were mounted with VECTASHIELD® Antifade Mounting Medium with DAPI (catalog number H-1200, Vector Laboratories, Burlingame, CA, USA). Images were capture using ZEISS Apotome.2 microscope and processed with Adobe Photoshop CC 2019 (Adobe Systems, Mountain View, CA, USA).
RESULTS
Genetic findings
Genetic studies were performed in nine families with one or more individuals affected with moderate–severe ID, speech delay, and nonspecific facial dysmorphisms. Additionally, some individuals presented with behavioral problems such as aggressiveness and shy character, body tremors, decreased muscle mass in lower extremities, seizures, and hypotonia. Detailed clinical descriptions are provided in Table 1 and in the Supplemental data.
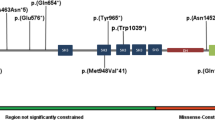
Exome sequencing was performed for the proband/index of each family (Supplemental data). All TMEM222 (GenBank: NM_032125.2) variants were confirmed and their familial segregation was validated by Sanger sequencing. In family 1, a homozygous c.535_537del deletion (p.[Val179del]) was identified in TMEM222 (Fig. 1a, Table 1). In family 2, we found a homozygous c.214G>A (p.[Gly72Ser]) missense variant, in family 3 we identified a homozygous c.526G>A (p.[Gly176Arg]) missense variant, and in family 6 we found the homozygous c.442G>A (p.[Val148Met]) variant, all predicted to be deleterious (CADD score >29)27. In family 4 we found a homozygous c.447_468del (p.[Leu150Thrfs*10]) frameshift deletion; in family 5, we identified the compound heterozygous splice-site variants c.279 + 2T>C and c.280-2A>T, affecting the strictly conserved nucleotides at the splice donor and acceptor site, respectively. In family 7 we found the homozygous splice variant c.539 + 2T>C affecting the strictly conserved nucleotides at the splice donor site. In family 8 we identified an insertion in exon 2 of TMEM222 (c.207dup), which creates a frameshift starting at codon Ile70 resulting in termination after 47 amino acids (p.[Ile70Hisfs*47]). In family 9 we found the homozygous c.334C>T (p.[Gln112*]) variant leading to a premature stop codon. Predictions indicate messenger RNA (mRNA) could be degraded via nonsense‐mediated mRNA decay (NMD), resulting in absence or an abnormally sized protein lacking the C-terminal part of the DUF778 domain and downstream amino acids.
(a) Pedigrees of families and clinical appearance of affected individuals. Arrows show patients described on Table 1. (b) Phenotypic features of affected individuals. (c) Schematic representation of human TMEM222 including the positions and the predicted effect on protein level of the eight identified variants in this study. Dark gray box represents the domain of unknown function DUF778 (positions 61–178). Dashed boxes represent exons. Black and light gray boxes represent the position of the protein in the membrane; IN inside (positions 77–164 and 208), OUT outside (positions 1–55 and 186), TM transmembrane (positions 56–76, 165–185, and 187–207). (d) Clustal W39 amino acid sequence alignment of TMEM222 from human, mouse, zebrafish, C. elegans, and fruit fly. Square dashed boxes indicate the position of the mutated amino acid conserved.
Sanger sequencing showed that these variants were present in a recessive manner in the affected individuals and inherited from heterozygous parents. Unaffected siblings had either two wild-type alleles or were heterozygous for the familial TMEM222 variant (Fig. 1b). None of these variants were present in the Genome Aggregation Database in a homozygous state (gnomAD; https://gnomAD.broadinstitute.org/)28.
Clinical spectrum
All affected individuals presented with moderate to severe ID, motor delay, and no speech/speech delay (Table 1). Variable clinical manifestations include nonspecific facial abnormalities (N = 10/17, 59%; Fig. 1b), hypotonia (N = 10/15, 67%), broad gait (N = 5/12, 42%), seizures (N = 7/17, 41%), abnormalities in the magnetic resonance image (MRI) (N = 5/8, 62%; Supplemental Fig. S2), and neuropsychiatric problems characterized by aggressive behavior (N = 6/17, 35%). Five patients presented with body tremors, three patients had decreased muscle mass in lower extremities, and two patients presented with disorder of the motor neurons. Patients in family 3 presented with macrocephaly (IV:6 + 1.32 SD, IV:7 + 2.54 SD). Patients from family 2 (IV:2 -2.09 SD, IV:3 -3.31 SD, IV:13, -3.31 SD, IV:14 -3.32 SD), family 4 (IV:2 -2.81 SD), and family 7 (IV:2 -3.91 SD) presented with microcephaly and secondary microcephaly was observed in individual V:1 from family 6 (-2 SD). In the rest of the patients studied the head circumference was in normal range. No striking growth abnormalities or common characteristic dysmorphic features were present. Detailed clinical phenotypes of all patients are provided in the Supplemental data. The clinical phenotype of heterozygous individuals was normal.
TMEM222 expression studies
Transmembrane protein 222, encoded by TMEM222, is comprised of 208 amino acid residues, and has a domain of unknown function (DUF778); however, the function of this protein has not yet been elucidated. To gain more insight into the role of TMEM222, we measured the gene expression level compared with three housekeeping genes (GUSB, PPIB, and CLK2)24 in several human tissues. Our results show that TMEM222 is highest expressed in brain with ubiquitous but lower expression levels in non-neuronal tissues (Fig. 2). Similarly, TMEM222 expression levels retrieved from the Genotype-Tissue Expression Project29 (GTEx, Supplemental Fig. S3) show a relatively high expression levels in cerebellum, cerebellar hemisphere, and tibial nerve, supporting a more specific role for TMEM222 in neurodevelopment.
To characterize the cellular localization of the protein, we used a commercially available polyclonal antibody for human TMEM222 (catalog number NBP2-49295, Novus Biologicals). Western blot analysis of HEK293T cells transfected with expression vector pcDNA3native/DEST-TMEM222 revealed that the anti-TMEM222 polyclonal antibody specifically identifies a 23.2-KDa protein that was less abundant/not detectable in mock-transfected cells (Supplemental Fig. S1). Next, immunocytochemistry was used to test the endogenous expression of TMEM222 in several cell lines. The highest TMEM222 levels were detected in the mature human iPSC-derived glutamatergic cortical neurons (at DIV28), briefly iNeurons, whereas moderate levels were detected in immature iNeurons (DIV10) and SK-N-SH neuroblastoma cells (Fig. 3a), and very low levels in HEK293T, fibroblasts, and hTERT RPE-1.
Due to the unknown function of the protein, we used PSORT II (https://psort.hgc.jp/form2.html) to predict the subcellular localization sites of the protein. TMEM222 is predicted to localize in vesicles of the secretory system30. Considering that TMEM222 has a high abundance in the brain and mature human iNeurons, and appears to play a role in vesicles, we sought to investigate the localization of TMEM222 in mature iNeurons. The TMEM222 antibody showed expression of the protein concentrated within the dendrites in a punctate pattern (Fig. 3b). We hypothesized that this expression could be in synaptic vesicles within the neuron. To test this, we performed colocalization analysis with different markers: TMEM222 colocalized with MAP2 and partially colocalized with postsynaptic marker (PSD95), early endosomal and ER exit markers (EEA1, RAB5, KIF16B, and SAR1A respectively; Fig. 3b). These data show that TMEM222 is localized in the dendrites and might play a role in postsynaptic vesicles in neurons.
DISCUSSION
In this study, we identified four missense homozygous variants, two frameshift homozygous variants, three splice-site variants, and one nonsense variant in TMEM222, in nine unrelated families, with a total of 17 affected individuals presenting with autosomal recessive ID and variable associated phenotypes, such as aggressive behavior, shy character, body tremors, decreased muscle mass in the lower extremities, and mild hypotonia.
It is interesting to note that interrogation of the gnomAD database28 shows that TMEM222 is a missense variation tolerant gene (Z-score = 0.44, probability of loss of function intolerance [pLI] = 0). In our study we found recessive variants in TMEM222; in this case heterozygous variants could be tolerated but homozygous ones are not. Even though the tolerance scores for this gene are low, we noticed that only two coding missense variants and no loss-of-function variants that were present in the gnomAD database were found in a homozygous state. Additionally, no homozygous variants with low allele frequency (<0.01) were found in Bravo (https://bravo.sph.umich.edu/freeze5/hg38/), a variant browser with variants observed in 62,784 individuals. Both the available data from gnomAD and the recessive TMEM222 variants identified (across multiple affected families) in this study suggest this gene may tolerate heterozygous variants but is not tolerant to biallelic variants.
All variants reported in this study are predicted to be deleterious (Table 1), but there are no known 3D structure or modeling templates available for TMEM222 to predict the effects of amino acid changes on TMEM222 protein structure. Considering that nonsense variants lead to degradation of mRNA via NMD31,32, the loss-of-function variants found in families 4, 5, 7, 8, and 9 are predicted to undergo NMD, resulting in absence or an abnormally sized protein lacking the C-terminal part of the DUF778 domain and downstream amino acids. Additionally, based on protein sequence predictions and annotations publicly available, missense and in-frame variants are conserved across species (Fig. 1d) and the missense variants are predicted to affect protein function (Table 1, CADD score >29)33. The in-frame deletion p.(Val179del) found in family 1 is predicted to be deleterious and disrupt the interactions of the transmembrane helix. The p.(Gly72Ser) variant found in family 2 likely disrupts protein localization due to the size of amino acid change. The p.(Gly176Arg) variant identified in family 3 is predicted to introduce a charged arginine in the transmembrane region, which would similarly interfere with its membranous localization. Finally, the p.(Val148Met) change found in family 6 might disturb the protein interactions, or the local core of the domain might be less stably folded, due to the larger size of the changed amino acid (http://lbqp.unb.br/NetWheels/).
The clinical symptoms in the patients, including severe developmental delay, hypotonia, significant speech delay, decreased muscle mass in lower extremity, neuropathy, secondary microcephaly, and other neurological symptoms such as tremor and lack of coordination and seizures, may suggest a neurodegenerative process, but a careful clinical reanalysis and longitudinal assessment of patients is required to establish this. In general, the patients presented here show significant clinical variability, which appears to be stronger between patients from different families than for affected individuals within the same family (Table 1). An explanation for this could be the differential functional consequences of the various allelic variants. In contrast to the clear loss-of-function frameshift, nonsense, and splice-site variants, some missense variants may be hypomorphic alleles capable of producing protein with residual activity. Another explanation for the clinical variability might be the presence of additional recessive hits. In three families, additional recessive variants were identified. In family 1, affected patients have a recessive missense variant passing our filtering criteria in SHISA6 (c.1043C>T, p.[Pro348Leu]; Table 1), a gene previously reported to have a critical role in maintenance of high-frequency synaptic transmission at hippocampal synapses34. This missense variant is found present 531 times in the gnomAD database, and four homozygotes. No clinical phenotype has been reported in individuals carrying pathogenic variants this gene. In family 3, affected individuals have recessive variants in three other genes (Table 1, Supplemental data): a splice-site variant c.6-1G>A in RGMB, and missense variants c.110C>T, p.(Thr37Met) in BSDC1 and c.294C>A, p.(His98Gln) in NDUFS5. The splice-site variant in RGMB is indicated in gnomAD as a possible annotation and is homozygously present 27 times in the database. None of these three genes have previously reported variants leading to a clinical phenotype. A possible additive clinical effect cannot be excluded for any of these homozygous variants, but the features of the patients from this family are not supportive of this possibility. In the patient of family 5, a variant of unknown significance paternally inherited was identified in the NF1 gene, which may add to the severity of the phenotype in this case. Finally, the clinical variability seen for the patients in this study might also be due to (rare) variants in coding or noncoding regions affecting genes that influence the TMEM222 phenotype. Further possible contributions to the phenotypes observed in this study might also be due to the effects of modifying genes that influence the TMEM222 phenotype.
The relatively high abundance of TMEM222 in the brain, especially the parietal and occipital cortex, together with the high protein expression in mature human iNeurons and the probable localization to early endosomes and in synapses, support a more specific role for TMEM222 in neurophysiological processes. The proteins of the TMEM family are predicted to be components of various cell membranes, such as mitochondrial, lysosomal, Golgi, and endoplasmic reticulum membranes. TMEM222 is predicted to encode for a transmembrane protein, according to the Pfam database22, and is highly expressed in brain with ubiquitous but lower expression levels in non-neuronal tissues (Fig. 2). Moreover, anti-TMEM222 antibody revealed the highest TMEM222 levels detected in the mature iNeurons and colocalization with MAP2 and partial colocalization with postsynaptic marker (PSD95), and early endosomal and ER exit markers (EEA1, RAB5, KIF16B, and SAR1A respectively; Fig. 3b), suggesting a role in postsynaptic vesicles in neurons.
UniProtKB33 lists several possible TMEM222-interacting proteins with a membranous localization. These interacting proteins have various cellular roles, including endosomal trafficking, synaptic vesicle shuttling, sterol metabolism, and mitochondrial calcium influx. For some of these proteins, variants have been identified in the corresponding gene that are associated with phenotypic features that overlap those associated with TMEM222 variants reported here. Biallelic variants in TMX2 cause a neurodevelopmental disorder (NEDMCMS; OMIM 618730) characterized by severe to profound global developmental delay, early-onset seizures, microcephaly, and polymicrogyria and/or cerebral atrophy. Most affected individuals are unable to walk or speak and have profound ID as well as axial hypotonia and peripheral spasticity. TMX2 is an ER protein that functions in post-translational protein modification and folding, and mitochondrial calcium flux and cellular redox homeostasis35. Hemizygous variants in EBP cause MEND syndrome (OMIM 300960) with neurologic defects is an X-linked recessive disorder representing a continuous phenotypic spectrum including ID, short stature, scoliosis, digital abnormalities, cataracts, and dermatologic abnormalities. The variable manifestations of MEND syndrome are associated with a defect in sterol biosynthesis. Interestingly, several other TMEM222-interacting proteins, such as ERG28, ELOVL4, and COMT, are involved in sterol metabolic pathways. ELOVL4 is required for the synthesis of very long chain saturated fatty acids and very long chain polyunsaturated fatty acids uniquely present in retina, sperm, and brain36. Causative ELOVL4 variants have been identified in three different disorders, heterozygous frameshift and nonsense variants in Stargardt disease 3 (OMIM 600110), heterozygous missense variants in spinocerebellar ataxia 34 (OMIM 133190), and homozygous nonsense and frameshift variants in ichthyosis, spastic quadriplegia, and mental retardation (OMIM 614457). Phenotypic features associated with TMEM222 variants have significant overlap with the two latter conditions37,38, suggesting related biological functions in TMEM222 and ELOVL4.
In conclusion, we have identified 17 patients from nine unrelated families with deleterious autosomal recessive variants in TMEM222 presenting with moderate–severe ID, speech delay, and nonspecific facial dysmorphisms. Additionally, some individuals presented with behavioral problems such as aggressiveness and shy character, body tremors, decreased muscle mass in lower extremities, seizures, and hypotonia. Further functional studies are necessary to understand the pathophysiological mechanism of this new autosomal recessive ID syndrome.
Data availability
The TMEM222 variant data were submitted to LOVD database (https://www.lovd.nl/TMEM222) hosted at Leiden University Medical Center, the Netherlands. Accession numbers are available in Table 1. Additional data and materials, such as primer sequences and Sanger sequencing electropherograms, are available upon request.
References
American Psychiatric Association. Task Force on DSM-IV. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV. 4th ed. (American Psychiatric Association, Washington, DC, 1994).
Leonard, H. & Wen, X. The epidemiology of mental retardation: challenges and opportunities in the new millennium. Ment. Retard. Dev. Disabil. Res. Rev. 8, 117–134, https://doi.org/10.1002/mrdd.10031 (2002).
Ropers, H. H. & Hamel, B. C. X-linked mental retardation. Nat. Rev. Genet. 6, 46–57, https://doi.org/10.1038/nrg1501 (2005).
Maulik, P. K., Mascarenhas, M. N., Mathers, C. D., Dua, T. & Saxena, S. Prevalence of intellectual disability: a meta-analysis of population-based studies. Res. Dev. Disabil. 32, 419–436, https://doi.org/10.1016/j.ridd.2010.12.018 (2011).
Colantuoni, C., Purcell, A. E., Bouton, C. M. & Pevsner, J. High throughput analysis of gene expression in the human brain. J. Neurosci. Res. 59, 1–10 (2000).
Naumova, O. Y. U., Lee, M., Rychkov, S. Y. U., Vlasova, N. V. & Grigorenko, E. L. Gene expression in the human brain: the current state of the study of specificity and spatio-temporal dynamics. Child Dev. 84, 76–88, https://doi.org/10.1111/cdev.12014 (2013).
Musante, L. & Ropers, H. H. Genetics of recessive cognitive disorders. Trends Genet. 30, 32–39, https://doi.org/10.1016/j.tig.2013.09.008 (2014).
van Bokhoven, H. Genetic and epigenetic networks in intellectual disabilities. Annu. Rev. Genet. 45, 81–104, https://doi.org/10.1146/annurev-genet-110410-132512 (2011).
Jamra, R. Genetics of autosomal recessive intellectual disability. Med. Genet. 30, 323–327, https://doi.org/10.1007/s11825-018-0209-z (2018).
Ellison, J. W., Rosenfeld, J. A. & Shaffer, L. G. Genetic basis of intellectual disability. Annu. Rev. Med. 64, 441–450, https://doi.org/10.1146/annurev-med-042711-140053 (2013).
Riazuddin, S. et al. Exome sequencing of Pakistani consanguineous families identifies 30 novel candidate genes for recessive intellectual disability. Mol. Psychiatry 22, 1604–1614, https://doi.org/10.1038/mp.2016.109 (2017).
Kochinke, K. et al. Systematic phenomics analysis deconvolutes genes mutated in intellectual disability into biologically coherent modules. Am. J. Hum. Genet. 98, 149–164, https://doi.org/10.1016/j.ajhg.2015.11.024 (2016).
Antonarakis, S. E. Carrier screening for recessive disorders. Nat. Rev. Genet. 20, 549–561, https://doi.org/10.1038/s41576-019-0134-2 (2019).
Bamshad, M. J., Nickerson, D. A. & Chong, J. X. Mendelian gene discovery: fast and furious with no end in sight. Am. J. Hum. Genet. 105, 448–455, https://doi.org/10.1016/j.ajhg.2019.07.011 (2019).
Hamamy, H. et al. Consanguineous marriages, pearls and perils: Geneva International Consanguinity Workshop Report. Genet. Med. 13, 841–847, https://doi.org/10.1097/GIM.0b013e318217477f (2011).
Schmit, K. & Michiels, C. TMEM proteins in cancer: a review. Front. Pharmacol. 9, 1345, https://doi.org/10.3389/fphar.2018.01345 (2018).
Wrzesiński, T., Szelag, M. & Cieślikowski, W. A. et al. Expression of pre-selected TMEMs with predicted ER localization as potential classifiers of ccRCC tumors. BMC Cancer. 15, 518, https://doi.org/10.1186/s12885-015-1530-4 (2015).
Aruga, J. & Mikoshiba, K. Identification and characterization of Slitrk, a novel neuronal transmembrane protein family controlling neurite outgrowth. Mol. Cell. Neurosci. 24, 117–129 (2003).
Yuan, J. P., Zeng, W., Dorwart, M. R., Choi, Y.-J., Worley, P. F. & Muallem, S. SOAR and the polybasic STIM1 domains gate and regulate Orai channels. Nat. Cell. Biol. 11, 337–343, https://doi.org/10.1038/ncb1842 (2009).
Foulquier, F. et al. TMEM165 deficiency causes a congenital disorder of glycosylation. Am. J. Hum. Genet. 91, 15–26, https://doi.org/10.1016/j.ajhg.2012.05.002 (2012).
Brady, O. A., Zheng, Y., Murphy, K., Huang, M. & Hu, F. The frontotemporal lobar degeneration risk factor, TMEM106B, regulates lysosomal morphology and function. Hum. Mol. Genet. 22, 685–695, https://doi.org/10.1093/hmg/dds475 (2013).
El-Gebali, S. et al. The Pfam protein families database in 2019. Nucleic Acids Res 47, D427–D432, https://doi.org/10.1093/nar/gky995 (2019).
Sobreira, N., Schiettecatte, F., Valle, D. & Hamosh, A. GeneMatcher: a matching tool for connecting investigators with an interest in the same gene. Hum. Mutat. 36, 928–930, https://doi.org/10.1002/humu.22844 (2015).
de Brouwer, A. P. M., van Bokhoven, H. & Kremer, H. Comparison of 12 reference genes for normalization of gene expression levels in Epstein-Barr virus-transformed lymphoblastoid cell lines and fibroblasts. Mol. Diagn. Ther. 10, 197–204, https://doi.org/10.1007/BF03256458 (2006).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T]) method. Methods. 25, 402–408, https://doi.org/10.1006/meth.2001.1262 (2001).
Frega, M., van Gestel, S. H. C. & Linda, K. et al. Rapid neuronal differentiation of induced pluripotent stem cells for measuring network activity on micro-electrode arrays. J. Vis. Exp. 8, 54900, https://doi.org/10.3791/54900 (2017).
Rentzsch, P., Witten, D., Cooper, G. M., Shendure, J. & Kircher, M. CADD: predicting the deleteriousness of variants throughout the human genome. Nucleic Acids Res. 47, D886–D894, https://doi.org/10.1093/nar/gky1016 (2019).
Genome Aggregation Database Consortium, KarczewskiK. J. et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature. 581, 434–443, https://doi.org/10.1038/s41586-020-2308-7 (2020).
GTex Consortium. The Genotype-Tissue Expression (GTEx) project. Nat. Genet. 45, 580–585, https://doi.org/10.1038/ng.2653 (2013).
Nakai, K. & Horton, P. PSORT: a program for detecting sorting signals in proteins and predicting their subcellular localization. Trends Biochem. Sci. 24, 34–36 (1999).
Frischmeyer, P. A. & Dietz, H. C. Nonsense-mediated mRNA decay in health and disease. Hum. Mol. Genet. 8, 1893–1900, https://doi.org/10.1093/hmg/8.10.1893 (1999).
Maquat, L. E. When cells stop making sense: effects of nonsense codons on RNA metabolism in vertebrate cells. RNA. 1, 453–465 (1995).
UniProt: a worldwide hub of protein knowledge. Nucleic Acids Res. 47, D506–D515, https://doi.org/10.1093/nar/gky1049 (2019).
Klaassen, R. V. et al. Shisa6 traps AMPA receptors at postsynaptic sites and prevents their desensitization during synaptic activity. Nat. Commun. 7, 10682, https://doi.org/10.1038/ncomms10682 (2016).
Vandervore, L. V. et al. TMX2 is a crucial regulator of cellular redox state, and its dysfunction causes severe brain developmental abnormalities. Am. J. Hum. Genet. 105, 1126–1147, https://doi.org/10.1016/j.ajhg.2019.10.009 (2019).
Agbaga, M.-P., Brush, R. S., Mandal, M. N. A., Henry, K., Elliott, M. H. & Anderson, R. E. Role of Stargardt-3 macular dystrophy protein (ELOVL4) in the biosynthesis of very long chain fatty acids. Proc. Natl. Acad. Sci. U. S. A. 105, 12843–12848, https://doi.org/10.1073/pnas.0802607105 (2008).
Aldahmesh, M. A. et al. Recessive mutations in ELOVL4 cause ichthyosis, intellectual disability, and spastic quadriplegia. Am. J. Hum. Genet. 89, 745–750, https://doi.org/10.1016/j.ajhg.2011.10.011 (2011).
Cadieux-Dion, M. et al. Expanding the clinical phenotype associated with ELOVL4 mutation: study of a large French-Canadian family with autosomal dominant spinocerebellar ataxia and erythrokeratodermia. JAMA Neurol. 71, 470, https://doi.org/10.1001/jamaneurol.2013.6337 (2014).
Sievers, F. et al. Fast, scalable generation of high‐quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 7, 539, https://doi.org/10.1038/msb.2011.75 (2011).
Acknowledgements
We are grateful to the families who have participated in this study. This work was supported the EU FP7 Large-Scale Integrating Project Genetic and Epigenetic Networks in Cognitive Dysfunction (241995) (to H.v.B.), an ERC grant (to S.E.A.), National Institute on Neurological Disorders and Stroke (R01NS107428) (to Sheikh Riazuddin), the National Institute of Neurological Disorders and Stroke (NINDS) under award number K08NS092898, Jordan’s Guardian Angels and the Brotman Baty Institute (to G.M.M.) and state assignment of Ministry of Science and Higher Education of the Russian Federation for RCMG. D.L.P. is recipient of a CAPES Fellowship (99999.013311/2013-01). Part of this work was supported by Higher Education Commission of Pakistan (NRPU project number 10700 to M.S.).
Author information
Authors and Affiliations
Contributions
Conceptualization: D.L.P., M.A.F., P.M., H.v.B., Sheikh Riazuddin. Data curation: D.L.P., A.P.M.d.B., H.v.B. Formal analysis: D.L.P., H.V., H.v.B., N.N.K. Methodology: D.L.P., A.P.M.d.B., H.V., K.L., F.F., N.F., O.A.L., P.N., P.M., M.H., S.v.H., A.K.B., P.H., Z.T., S.Z., M.S.F., J.H.M.S.H., M.S., L.D.K., H.T.B., L.R.F., J.C., A.P., J.P., N.A.D., A.L.C., V.S.S., A.T.M., N.N.K., H.H., F.A.S., Z.M.A., S.H., M.Y., M.A., M.S. G.R., E.E., A.T.S., S.B., G.M.M. Supervision: M.A.F.F., R.P., Saima Riazuddin, Sheikh Riazuddin, F.P., A.V.L., S.E.A., M.A.F., H.v.B. Validation: D.L.P., H.v.B. Visualization: D.L.P., P.M., H.v.B. Writing—original draft: D.L.P., H.v.B. Writing—review & editing: D.L.P., M.A.F.F., R.P., Saima Riazuddin, Sheikh Riazuddin, F.P., A.K.B., A.V.L., S.E.A., M.A.F., H.v.B.
Corresponding author
Ethics declarations
ETHICS DECLARATION
Individuals were identified in different centers worldwide in diagnostic or research settings approved by the respective institutional review boards: the Institutional Review Board Commissie Mensgebonden Onderzoek Regio Arnhem-Nijmegen, the Netherlands; Institutional Review Board at University of Maryland School of Medicine, Baltimore, Maryland, USA; Institutional Review Board, Allama Iqbal Medical College, University of Health Sciences, Lahore, Pakistan; Institution Board of Jordan University Hospital, Amman, Jordan; Bioethics Committee of the University Hospitals and the Canton of Geneva, Switzerland; Ethics Committee of the Research Centre for Medical Genetics, Moscow, Russia; Persian BayanGene Research and Training Center Ethics Committee, Shiraz, Iran; institutional review boards of the Hanover Medical School the Children’s and Youth Hospital Auf der Bul, Hannover, Germany. Written informed consents were obtained from all the participating adults and guardians of affected individuals. Specific written informed consent was obtained for publishing the patients’ photographs. This study adhered to the World Medical Association Declaration of Helsinki (2013).
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Polla, D.L., Farazi Fard, M.A., Tabatabaei, Z. et al. Biallelic variants in TMEM222 cause a new autosomal recessive neurodevelopmental disorder. Genet Med 23, 1246–1254 (2021). https://doi.org/10.1038/s41436-021-01133-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41436-021-01133-w