Abstract
Purpose
Pathogenic variants in STUB1 were initially described in autosomal recessive spinocerebellar ataxia type 16 and dominant cerebellar ataxia with cerebellar cognitive dysfunction (SCA48).
Methods
We analyzed a large series of 440 index cerebellar ataxia cases, mostly with dominant inheritance.
Results
STUB1 variants were detected in 50 patients. Age at onset and severity were remarkably variable. Cognitive impairment, predominantly frontal syndrome, was observed in 54% of STUB1 variant carriers, including five families with Huntington or frontotemporal dementia disease–like phenotypes associated with ataxia, while no STUB1 variant was found in 115 patients with frontotemporal dementia. We report neuropathological findings of a STUB1 heterozygous patient, showing massive loss of Purkinje cells in the vermis and major loss in the cerebellar hemispheres without atrophy of the pons, hippocampus, or cerebral cortex. This screening of STUB1 variants revealed new features: (1) the majority of patients were women (70%) and (2) “second hits” in AFG3L2, PRKCG, and TBP were detected in three families suggesting synergic effects.
Conclusion
Our results reveal an unexpectedly frequent (7%) implication of STUB1 among dominantly inherited cerebellar ataxias, and suggest that the penetrance of STUB1 variants could be modulated by other factors, including sex and variants in other ataxia-related genes.
Similar content being viewed by others
INTRODUCTION
The STUB1 gene (STIP1 Homology And U-Box Containing Protein 1) encodes the CHIP protein containing tetratricopeptide repeats and a U-box that functions as a ubiquitin ligase/cochaperone, involved in the cellular protein quality control system. In 2013, homozygous and compound heterozygous variants in STUB1 were detected in three families.1 Subsequently, it was demonstrated that STUB1 pathogenic variants caused autosomal recessive spinocerebellar ataxia type 16 (SCAR16).2 Cognitive impairment has been described in several STUB1 patients, either inaugural or up to 20 years after onset.3 Variants in this gene were further described in a dominant form of spinocerebellar ataxia, named SCA48.4 Moreover, a cerebellar cognitive affective syndrome (CCAS), described in patients with lesions confined to the cerebellum, was detected in this family.5,6
Novel STUB1 pathogenic variants in two and eight Italian families respectively with a complex syndrome characterized by ataxia and cognitive–psychiatric disorder have been separately reported by the same group.7,8 Thus, STUB1 has been linked to either recessive or dominant forms of ataxia with cognitive impairment and, in a single family, associated with hypogonatropic hypogonadism. We screened a large series of families with cerebellar ataxia, frontotemporal dementia (FTD), or Huntington-like disease for STUB1 variants.
MATERIALS AND METHODS
Screening of patients with cerebellar ataxia
In total, 440 families, including 491 patients were recruited as part of the SPATAX cohort (https://spatax.wordpress.com/). They were examined by at least one member of the SPATAX network listed at the end of the paper and clinically assessed using a standardized evaluation form (https://spatax.files.wordpress.com/2013/09/fichecliniquespatax-eurospa-2011.pdf). Autosomal dominant inheritance was defined by the presence of at least one other affected individual among parents or children of the index case in 320 cases. Sixty-two families were suggestive of a recessive model of inheritance (32 with reported consanguinity), and 58 were “isolated” cases. All patients gave written informed consent, and blood samples were collected in accordance with local French regulations (Paris Necker ethics committee approval [RBM 01–29 and RBM 03–48] to A.B. and A.D.). DNA was extracted using classical procedures. Polyglutamine expansions in ATXN1, 2, 3, 7, TBP, CACNA1A, and ATN1 were excluded in all patients.
FTD and Huntington-like disease cohort
The 115 FTD patients were consecutively recruited by experts from the French clinical and genetic research network on FTD/FTD–amyotrophic lateral sclerosis (ALS). The diagnoses of FTD were based on international diagnostic criteria.9,10 We also included four cases that did not have expansions in HTT or JPH3 or variants in other genes included in a panel that we used to identify the genetic cause of Huntington-like disease cases.11
Genetic analysis
A screening of STUB1 genetic variants was performed on 440 index cases who had previously undergone a gene panel screening, of which 324 have been reported in Coutelier et al.12 The remaining families underwent exome sequencing (n = 111) or genome sequencing (n = 5). The mean coverage of STUB1 in the ataxia cohort was 230× and >20× for all samples. Rare variants predicted to be pathogenic were filtered (minor allele frequency [MAF] <0.001 in the gnomAD database 2.1.1 (https://gnomad.broadinstitute.org/); Combined Annotation Dependent Depletion [CADD phred] score >20).13 All variants were validated by Sanger sequencing. The segregation of STUB1 pathogenic variants was performed in ten families in which at least two individuals were available. Primers used to confirm variations and for segregation analyses are presented in Supplementary Table 1.
The STUB1 gene was analyzed by exome sequencing in 115 patients with FTD. The mean coverage of STUB1 in the FTD cohort was 46.9×, and >20× for all samples. The same filtering of rare variants used in the ataxia cohort was also applied to the four additional HD-like cases in which the mean coverage of STUB1 was 113× and >20× for all patients.
Neuropathology
A systematic sampling of the cerebellum, brainstem, and hemisphere was performed. Three-micron-thick sections were deparaffinized and stained with hematein–eosin. Slides were immune-stained with a Ventana-Roche BenchMark ULTRA IHC/ISH System. The following antibodies were used: antiubiquitin (polyclonal, Dako), p62 (3/p62 LCK Ligand, BD Transduction Laboratory), polyglutamine (1C2, Euromedex), TDP43 (polyclonal, Euromedex), phosphorylated TDP43 (1D3, Millipore), Aβ (6F3D Dako), and Tau (AT8 Thermo Electron).
Ethics statement
All patients gave written informed consent, and blood samples were collected in accordance with local French regulations (Paris Necker ethics committee approval [RBM 01–29 and RBM 03–48] to A.B. and A.D., CPP Ile de France II ethics committee approval [RBM 02–59] to Isabelle Le Ber). All written patient consents were archived. Brain removal was performed as part of the program “Brain Donation for Research” (National Neuro-CEB Brain Bank, GIE Neuro-CEB BB-0033–00011).
RESULTS
Screening of STUB1 variations in SCA, FTD, and HD-like cohorts
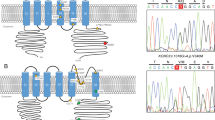
We screened 440 families who previously underwent exome sequencing or gene panel analysis for STUB1 genetic variants. Twenty-six different variants were observed in 30/440 families, of which 22 were absent from and 5 were ultrarare in public databases, predicted to be deleterious (Table 1). The STUB1 variants were mostly missense pathogenic variants, along with a number of substitutions, including stop-gains, and frameshift variants (Table 1). The mean CADD score was 29.67 (from 23.1 to 43). Four were previously reported in patients with either SCAR16 or SCA48 and four new variations affected amino acids that have been previously shown to be mutated and associated with SCAR16 or SCA48,1,4,8,12,14,15,16,17 (Table 1, Fig. 1). Variants were located throughout the coding sequence, without evidence of any genotype–phenotype correlation (Fig. 1). Of note, five STUB1 variants were recurrent in unrelated families, all absent from the gnomAD database or ultrarare.
The CHIP protein (NP_005852.2) harbors three-tetratricopeptide repeat domains (TPR1–3) and a U-box domain acting as a ubiquitin ligase. Variants detected in our cohort are indicated according to their location on the protein sequence. Novel variants are shown above the protein structure and variants that have been described elsewhere are shown below. In italics: amino acid positions already associated with SCA48 or SCAR16 elsewhere. In green: substitutions with recessive inheritance. In red: amino acid changes detected in unrelated patients.
Clinical data of 50 patients harboring STUB1 variants are presented in Table 2; most were French, with the exception of 3 Belgian, 2 Italian, 1 German, and 1 British. Most patients were women (36/50; 72%). Inheritance was dominant in 23 families, transmission mostly by affected mothers and in four families only one generation was affected, with several affected sibs and three isolated cases. The mean age at onset was 40.0 ± 13.8 years, ranging from 17 to 74, and was similar in 29 women and 11 men (44.1 ± 13.7 vs. 39.3 ± 11.6, p = 0.310). In heterozygous carriers, the age at onset ranged from 23 to 74 years with high variability. In one family (SAL-338), the heterozygous father was unaffected at 65 years of age indicating age-dependent penetrance.
Symptoms at onset were unsteadiness in most (79%; 39/49), associated with dysarthria in 46% (18/39). Strikingly, cognitive impairment, mostly dysexecutive, was observed and sometimes predominant in 54% (26/48) of the patients. One patient with intellectual deficit (SAL-399–729–1) harbored compound heterozygous variants. The evaluation of cognitive impairment by the neurologists at bedside concluded to the presence of a frontal syndrome with predominant behavioral changes for 14/26 patients (disinhibition, aggressiveness) but no evident aphasia, memory loss, or apraxia. These behavioral changes were reminiscent of what is generally observed in patients with Huntington disease (HD) and indeed, HD was suspected in three families based on clinical symptoms (Table 2). Pyramidal signs were reported in 43% of patients. There was no wasting or sensory loss. Movement disorders such as myoclonus, dystonic postures or chorea, were present in 57%. Diplopia, ptosis, nystagmus, or saccadic pursuit was sometimes present but ophthalmoplegia was rare. The stage of disability varied from 1 to 6. Patients with onset before age 40 (n = 14) showed a more severe functional involvement than those with later onset (disability stage 3.1 vs. 2.8, p = 0.008), although with a longer follow-up in patients affected before 40 years of age (9.7 vs. 7.1 years, p = 0.45).
The most significant difference appeared to be between patients from two families with recessive inheritance (one compound heterozygote and one homozygote STUB1 variant carrier) relative to those with dominant inheritance. Patients biallelic for STUB1 variants were younger at onset (19.3 vs. 42.4, p < 0.05) and their symptoms tended to be more severe than those who were heterozygous (disability stage 5.3 vs. 2.9, p = 0.40, despite a similar disease duration of 8.2 vs. 10 years). However, cognitive impairment was observed in both groups.
Given the cognitive phenotype of patients with STUB1 pathogenic variants, we analyzed exome sequencing data from 115 patients with FTD for pathogenic variants in this gene. The same criteria for variants selection were applied. For 13 patients, an FTD gene had been previously identified (C9orf72, GRN, MAPT, TARDPB, and VCP). Contrary to what was observed in the patients with ataxia, we detected no potential STUB1 candidate variant in the FTD patients. Overall, these findings favor the strong enrichment of rare STUB1 variations in SCAs relative to FTD (30/440 vs. 0/115, Fisher’s exact test p = 0.0017). No variant was found in the exome sequencing data of four HD-like families, for which there was no ataxia associated with chorea.
Aside from the identification of STUB1 pathogenic variants in exome sequencing or gene panels, we detected potential second hits in five families. In one of the two families carrying STUB1 p.Y49C (AAD-541, Supplementary Fig. 1A), a previously unreported variant of AFG3L2 (rs753000167, p.A484P, CADD = 33, MAF gnomAD < 1.10e-5), a gene associated with SCA28, was found to segregate with the disease (Supplementary Fig. 1A). However, the phenotype of this family is not typical of SCA28, given the cognitive impairment and lack of ophthalmoplegia. In addition, the same STUB1 variant was found in another family (SAL-2352). This favors STUB1 as the causal gene. Nevertheless, it cannot be ruled out that the AFG3L2 variant, which cosegregates with the STUB1 variant, could contribute to the phenotype.
In a second family (AAD-452, Supplementary Fig. 1C), a variant was found in PRKCG (SCA14, p.H347R, CADD = 20.3, absent from gnomAD). We previously published this substitution as a variant of unknown significance (VUS).12 The additional STUB1 variant found in this patient (p.N65S) has already been associated with SCAR16 and described to impair the ability of CHIP to ubiquitinate heat shock protein 70 in vitro and to decrease CHIP protein levels in the fibroblasts of variants carriers.16
Two families (AAD-075 and AAD-391) were previously screened for the CAG/CAA repeats in TBP (SCA17) and found to have a number of repeats close to or at the threshold of a positive diagnosis for SCA17. Probands of families AAD-075 (STUB1 p.A113D, CADD = 29; absent from gnomAD) and AAD-391 (STUB1 p.R154C, CADD = 28.2, absent from gnomAD) carried 41 and 46 CAG/CAA repeats, respectively. It is known that the CAG/CAA repeat length in the TBP gene is highly variable and the number of glutamines encoded by CAG/CAA repeats ranges from 25 to 42 in the US population, excluding SCA17 in family AAD-075.18 The detection of 46 repeats in cases from family AAD-391 and the presence of positive neuronal intranuclear inclusions containing polyglutamines in a neuropathological case led us to previously consider this family as SCA17.19 However, there is growing evidence that not only the size of the CAG/CAA repeat but also the size of the pure (CAG)n play a major role in defining the pathological threshold.20 Therefore, the TBP expansion in family AAD-391 may be causal or act in concert with the STUB1 variant (Supplementary Fig. 1B).
Finally, we detected a second homozygous variant in family AAR-030, in accordance with the supposed mode of inheritance. This substitution, located in ATM (rs587779876, p.E299G), was reported as a VUS in the ClinVar database. Moreover, the homozygous p.L231V STUB1 variant explains the phenotype of ataxia with hypogonadism, Gordon Holmes–like syndrome. The transmitting parent and grandparent carried the STUB1 variant without obvious ataxic or cognitive impairment at age 58 and 72, respectively. In addition, we did not detect any elevation of alpha-fetoprotein, the hallmark of ataxia telangiectasia, or immunological disorder, ruling out ATM as a causal gene in this family.
Case reports
Family AAD-541 (Supplementary Fig. 1A)
The index case, a 70-year-old man, presented with psychiatric symptoms, such as aggressive behavior, emotional indifference, and apathy at age 40. At first neurological examination at age 53, the Mini-Mental State Examination (MMSE) was 24/30 and a frontal syndrome was noted. There was no apraxia, aphasia, or memory loss. Neurological examination revealed an ataxic gait, dysmetria of the upper limbs, and cerebellar dysarthria. He stopped working at age 54. On follow-up at age 70, neuropsychological evaluation showed a severe CCAS. The frontal syndrome was at the forefront, with dorsolateral and orbitofrontal features. The patient had a severe dysexecutive syndrome (BREF 6/18) and affective deficits, with behavioral impairment and irritability. Moreover, the evaluation underlined attentional impairment with working memory deficits. Neurological examination showed ataxic gait, dysmetria of the four limbs, oculomotor signs with a saccadic pursuit and multidirectional nystagmus, and severe dysarthria without aphasia. The Scale for the Assessment and Rating of Ataxia (SARA) score was 26 (maximum worse score 40). There was no ptosis or ophthalmoplegia, or pyramidal syndrome or motor deficit. Cerebral magnetic resonance image (MRI) showed global cerebellar atrophy (Fig. 2).
The symptoms of his daughter, a 43-year-old woman, started at age 38 with dysarthria. She experienced a depressive syndrome for two years. Neuropsychological evaluation showed clear dysexecutive syndrome with a deficit in lexical access and slower information processing. Orbitofrontal features were also present, with apathy and emotional recognition impairment. Spatial cognition deficits were noted, with visual construction impairment. Other cognitive functions were preserved. Ocular movement recordings showed no cerebellar features but an increase of errors in the antisaccades test, suggestive of a frontal syndrome. There was no ptosis, ophthalmoplegia, or pyramidal signs. Her cerebral MRI also showed clear cerebellar atrophy (Fig. 2). As mentioned above a variant in AFG3L2 (p.A484P) was reported, as well as a variant in STUB1 (p.Y49C), which both segregated with the disease (Supplementary Fig. 1A).
Family SAL-2352
The index case was a 51-year-old woman at onset with behavior impairment, emotional lability, and irritability characterized as frontal syndrome. She presented with chorea associated with mild dystonia, mild plastic rigidity, and mild gait ataxia. Reflexes were elevated in the lower limbs without a Babinski sign. The first hypothesis was HD but genetic testing for CAG repeat expansion in HTT was normal. She was re-evaluated two years later and showed additional unsteadiness and dysarthria.
Her niece presented with similar symptoms with onset at age 37, mild chorea, myoclonus, and plastic rigidity in the upper limbs. A cerebellar syndrome was observed with unsteadiness, dysarthria, saccadic pursuit, slow saccades, and oculomotor apraxia. Her SARA score was 12.5/40. Behavioral changes were also present. In this family, we detected the same STUB1 variant (p.Y49C) as that in family AAD-541 was detected, these two family being unrelated.
Neuropathological case (family SAL-345): clinical and neuropathological description
A second family presented with a Huntington-like phenotype. The index case reported irritability and aggressiveness with onset at 47 years of age. Her clinical examination also showed diffuse chorea, facial dystonia, mild rigidity in the right upper limb, and a cerebellar syndrome, with unsteadiness and dysarthria. She died at age 62 after severe status epilepticus secondary to brain trauma after a fall from her height. The brain was examined (Fig. 3).
(a–d) Hematoxylin–eosin staining showing cerebellar changes. (a) Vermis showing the loss of Purkinje cells and prominent Bergmann glia. The Purkinje cell layer is indicated by an asterisk. Two empty baskets are shown by the arrowheads and prominent Bergmann glia by arrows. Scale bar = 50 µm. (b) Vermis showing the loss of granule neurons, responsible for the spongiotic aspect of the granular layer (e.g., in the region indicated by the arrows). Scale bar = 50 µm. (c) Cerebellar hemisphere, showing a pyknotic nucleus in a Purkinje cell (arrow). Scale bar = 20 µm. (d) Cerebellar hemisphere showing a “torpedo” (arrow) below the cell body of a Purkinje cell. Scale bar = 20 µm. (e, f) Hematein–eosin staining of the dentate nucleus and substantia nigra. (e) Dentate nucleus, showing numerous neurons visible (arrowheads). There is also evidence of astrogliosis (increased number of astrocytes with clear nucleus). Scale bar = 50 µm. (f) Substantia nigra, showing extraneuronal melanin pigments (arrows) and evidence of neuronal loss. Scale bar = 30 µm. (g, h) Hippocampus and neocortex. (g) AT8 tau immunohistochemistry showing two pretangles in the CA1 sector of the cornu ammonis. Scale bar = 30 µm. (h) Superior temporal gyrus (Brodmann area 22) showing normal numerical density of the neurons and type 2 Alzheimer gliosis: clear astrocytic nuclei are visible (arrow). Hematein–eosin stain. Scale bar = 30 µm.
Her sister presented with similar symptoms. Onset was reported at age 38. First examination at age 45 showed an extrapyramidal syndrome with hypokinesia and axial rigidity (without DOPA sensibility), facial chorea, cognitive impairment (MMSE 23/30, Mattis Dementia Rating Scale 120), a cerebellar syndrome with unsteadiness, saccadic pursuit, and dysarthria. She further developed scoliosis at age 55, a pyramidal syndrome in the lower limbs, with increased reflexes and moderate spasticity, and epilepsy at 64. Her brain MRI showed cerebellar atrophy, diffuse cortical atrophy, and atrophy of the pons. She died at age 73.
Her aunt also presented a cerebellar syndrome with unsteadiness and nystagmus with onset at age 60. In this family, exome sequencing revealed the presence of a STUB1 variant (p.A46P), currently absent from gnomAD with a CADD = 24.
The cerebellum was severely atrophic. The atrophy was severe in the vermis and less marked in the hemispheres. The inferior, middle, and superior cerebellar peduncles were normal, as well as the pontine nuclei. At microscopic examination, the loss of Purkinje cells was nearly complete in the vermis and extensive in the hemispheres; “empty baskets” were numerous. Bergmann glia were hyperplastic and hypertrophic. Axonal torpedoes were observed in the granular layer. Neuronal loss in the granular layer of the cerebellum was of variable severity predominating at the top of the lobules and immediately under the Purkinje cell layer. The dentate nucleus was normal. The olive was moderately affected with mild neuronal loss, and a few hypertrophic neurons, some being vacuolated (fenestrated); astrogliosis was marked. The presence of rare extrapyramidal pigments provided a direct evidence of mild neuronal loss in the substantia nigra. The subthalamic nucleus, the striatum, and the pallidum were normal. The hippocampus was macroscopically normal; at microscopic examination, the numerical density of neurons was normal. Tau (AT8) immunohistochemistry revealed infrequent pretangles and a few neuropil threads. The cerebral cortex was not atrophic. There was no neuronal loss. Rare p62 nuclear inclusions were seen in the frontal cortex and hippocampus (Fig. 3). No inclusions were seen after immunostaining with antibodies against polyglutamine (1C2 antibody), Tau (AT8), TDP-43 (polyclonal Euromedex), PrP (12F10), ubiquitin (polyclonal Dako), p62 (Clone 3/P62 Becton Dickinson), or α-synuclein (5G4).
DISCUSSION
We report a very large STUB1 screening of 440 ataxic families that revealed 26 variants of STUB1, in 30 families accounting for 7% of this cerebellar cohort. The pathogenic variants were all absent from public databases or ultrarare variants and predicted to be pathogenic.
Our study revealed an unexpectedly high rate of STUB1 variations in a large ataxia cohort. It is unlikely that we found rare variants in this gene by chance. First, we did not detect any rare variants in an additional series of 115 patients with FTD. Second, we selected STUB1 loss-of-function/missense variants with a CADD > 20 and a MAF below 0.001 in the gnomAD project v2.1.1/v3. In the gnomAD databases (both v2.2 and v3), the frequency of rare STUB1 variant carriers is ≈0.0014, which is significantly lower than in our ataxia cohort (chi-square test p < 0.0001). Third, we detected five recurrent pathogenic variants in unrelated patients with ataxia. These pathogenic variants are absent from gnomAD (p.N65S, p.C69Y, and pK143del) or very rare (p.Y49C and p.P243L, MAF ≤ 0.00003), the p.P243L and p.N65S variants having already been reported in SCAR16.16,21 Overall, there is a significant enrichment of rare STUB1 variants predicted to be pathogenic.
Strikingly, there was a clear deviation of the sex ratio among STUB1 patients and their transmitting parent, in favor of women (70% and 94%, respectively). This observation raises the question of the sex-dependent penetrance of STUB1 pathogenic variants. Of note, 48% (36/75) of women were asymptomatic among siblings in STUB1 families vs. 65% (30/46) in men (chi-square test p = 0.06). Although not all individuals were genotyped for STUB1 pathogenic variants, this observation is in line with the study of Lieto et al. In their study 8/11 patients were women, with a single case of incomplete penetrance in a male obligate carrier.8 A systematic screening of all relatives in STUB1 families would allow to confirm this hypothesis. Gene expression data for STUB1 in the GTEx database (GTEx Analysis Release V8; https://gtexportal.org/home/) show that, conversely to most noncerebral tissues tested, the expression of STUB1 tends to be lower in women than men in the brain, notably in the cerebellum. As CHIP levels are much lower in the fibroblasts of STUB1 variant carriers, women with lower amounts of CHIP in brain tissues may be more sensitive to haploinsufficiency. Heterozygous carriers showed significantly later onset and a less severe disease than compound heterozygous or homozygous STUB1 variant carriers. This suggests that the underlying mechanism is haploinsufficiency.
The fact that variants discovered in a recessive form of the disease give rise to a milder and later phenotype in a heterozygous state is reminiscent of variants of GRID2 and SPG7.22,23 For GRID2 pathogenic variants, the phenotype spans from mild adult onset in the heterozygous state, less pronounced in women, to congenital onset ataxia in the homozygous state. For SPG7, we described mildly affected parents from compound heterozygous children.
Very strikingly, 54% of patients who were evaluated presented with cognitive impairment. Dementia is rarely seen as a prominent sign in SCAs caused by CAG-triplet repeat expansions. In contrast, SCAs not caused by polyglutamine expansions show slower disease progression and cognitive impairment is more common.24 Frontal dysfunction has been observed in patients with only cerebellar lesions as described by Schmahmann concerning the features of the CCAS.5,6 The cerebellum has been shown to play a key role in social cognition and patients with cerebellar disorders find it difficult to attribute emotions to facial expression.25,26 Cognitive decline has been associated with STUB1 pathogenic variants.4,8 Cerebellar MRIs and neuropathological examinations did not reveal frontal or general cortical atrophy. Such preservation of the frontal lobe, in contrast to the severe atrophy of the cerebellum in both SCAR16 and SCA48 patients, favors CCAS.2,4,8,17 Moreover, the absence of any STUB1 variant in 115 FTD patients suggests a specific frontal lobe–like impairment associated with ataxia.
STUB1 pathogenic variants were detected in SCA patients without CAG repeat expansions in ATN1, ATXN1, ATXN2, ATXN3, CACNA1A, ATXN7, and TBP. In a few families, a potential second hit could have been involved in the disease as well. We found an additional STUB1 p.R154C variant in patient who carried 46 repeats in TBP. Interestingly, this variant segregated with the 46 CAG expansion in the aunt of our case in this large Belgian family (Supplementary Fig. 1B). Other DNA samples were not available. The neuropathology of the aunt, carrying both pathogenic variants, showed numerous 1C2 inclusions and major cerebellar atrophy with a normal pons.19 The latter phenotype was also found in our neuropathological case lacking inclusions.
Another intriguing case was the heterozygous p.N65S STUB1 variant detected in three families. This variant was already reported as causal leading to SCAR16 in three children from the same family, with disease onset before two years of age.16 Here, we show that heterozygous carriers may become symptomatic later with the age at onset ranging from 27 up to 55 years. Notably, the patient with the earliest age at onset, of 27 years, also harbored an additional PRKCG p.H347R variant (Supplementary Fig. 1C). Thus, such early onset may have been due to the synergistic action of two variants.
Previously published neuropathological features in a patient with recessive or dominant STUB1-linked ataxia were similar to ours.14,27 Major loss of Purkinje cells and a severe decrease in neurons of the granular layer in the cerebellum, but a preserved cerebral cortex were notably noticed. We could not confirm the STUB1 product mislocalization in absence of CHIP protein assessment. Cortical atrophy and nuclear inclusions were identified only in the family with the combined TBP/STUB1 pathogenic variants, probably due to CAG repeat expansion in TBP.19
This study strongly suggests that STUB1 genetic variations may represent a relatively common cause of dominant cerebellar ataxia, either alone or in combination with another ataxia-related gene variant. The frequent cognitive features (frontal syndrome and behavioral impairments as observed in HD) associated with cerebellar ataxia are a hallmark of this form. Thus, physicians should be aware of the involvement of STUB1 in families with the association of ataxia and cognitive decline.
Data availability
Anonymized data from this study will be shared by request from any qualified investigator.
Change history
04 December 2020
The original version of this Article contained an error in the author name for Lionel Van Maldergem. The family name was incorrectly spelt Van Maldergen. This has now been corrected in both the PDF and HTML versions of the Article.
24 February 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41436-020-01064-y
References
Shi Y, Wang J, Li J-D, et al. Identification of CHIP as a novel causative gene for autosomal recessive cerebellar ataxia. PLoS One. 2013;8:e81884.
Shi C-H, Schisler JC, Rubel CE, et al. Ataxia and hypogonadism caused by the loss of ubiquitin ligase activity of the U box protein CHIP. Hum Mol Genet. 2014;23:1013–1024.
Gazulla J, Izquierdo-Alvarez S, Sierra-Martínez E, Marta-Moreno ME, Alvarez S. Inaugural cognitive decline, late disease onset and novel STUB1 variants in SCAR16. Neurol Sci. 2018;39:2231–2233.
Genis D, Ortega-Cubero S, San Nicolás H, et al. Heterozygous STUB1 mutation causes familial ataxia with cognitive affective syndrome (SCA48). Neurology. 2018;91:e1988.
Schmahmann JD. The cerebellum and cognition. Neurosci Lett. 2019;688:62–75.
Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998;121(Pt 4):561–579.
De Michele G, Lieto M, Galatolo D, et al. Spinocerebellar ataxia 48 presenting with ataxia associated with cognitive, psychiatric, and extrapyramidal features: a report of two Italian families. Parkinsonism Relat Disord. 2019;65:91–96.
Lieto M, Riso V, Galatolo D, et al. The complex phenotype of spinocerebellar ataxia type 48 in eight unrelated Italian families. Eur J Neurol. 2020;27:498–505.
Gorno-Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–1014.
Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134(Pt 9):2456–2477.
Mariani L-L, Tesson C, Charles P, et al. Expanding the spectrum of genes Involved in Huntington disease using a combined clinical and genetic approach. JAMA Neurol. 2016;73:1105–1114.
Coutelier M, Coarelli G, Monin M-L, et al. A panel study on patients with dominant cerebellar ataxia highlights the frequency of channelopathies. Brain. 2017;140:1579–1594.
Kircher M, Witten DM, Jain P, O’Roak BJ, Cooper GM, Shendure J. A general framework for estimating the relative pathogenicity of human genetic variants. Nat Genet. 2014;46:310–315.
Bettencourt C, de Yébenes JG, López-Sendón JL, et al. Clinical and neuropathological features of spastic ataxia in a Spanish family with novel compound heterozygous mutations in STUB1. Cerebellum. 2015;14:378–381.
Hayer SN, Deconinck T, Bender B, et al. STUB1/CHIP mutations cause Gordon Holmes syndrome as part of a widespread multisystemic neurodegeneration: evidence from four novel mutations. Orphanet J Rare Dis. 2017;12:31.
Heimdal K, Sanchez-Guixé M, Aukrust I, et al. STUB1 mutations in autosomal recessive ataxias—evidence for mutation-specific clinical heterogeneity. Orphanet J Rare Dis. 2014;9:146.
Synofzik M, Schüle R, Schulze M, et al. Phenotype and frequency of STUB1 mutations: next-generation screenings in Caucasian ataxia and spastic paraplegia cohorts. Orphanet J Rare Dis. 2014;9:57.
Gostout B, Liu Q, Sommer SS. “Cryptic” repeating triplets of purines and pyrimidines (cRRY(i)) are frequent and polymorphic: analysis of coding cRRY(i) in the proopiomelanocortin (POMC) and TATA-binding protein (TBP) genes. Am J Hum Genet. 1993;52:1182–1190.
Fujigasaki H, Martin JJ, De Deyn PP, et al. CAG repeat expansion in the TATA box-binding protein gene causes autosomal dominant cerebellar ataxia. Brain. 2001;124(Pt 10):1939–1947.
Genetic Modifiers of Huntington’s Disease (GeM-HD) Consortium. CAG repeat not polyglutamine length determines timing of Huntington’s disease onset. Cell. 2019;178:887–900.e14.
Madrigal SC, McNeil Z, Sanchez-Hodge R, et al. Changes in protein function underlie the disease spectrum in patients with CHIP mutations. J Biol Chem. 2019;294:19236–19245.
Coutelier M, Burglen L, Mundwiller E, et al. GRID2 mutations span from congenital to mild adult-onset cerebellar ataxia. Neurology. 2015;84:1751–1759.
Klebe S, Depienne C, Gerber S, et al. Spastic paraplegia gene 7 in patients with spasticity and/or optic neuropathy. Brain. 2012;135(Pt 10):2980–2993.
Durr A. Autosomal dominant cerebellar ataxias: polyglutamine expansions and beyond. Lancet Neurol. 2010;9:885–894.
Hoche F, Guell X, Sherman JC, Vangel MG, Schmahmann JD. Cerebellar contribution to social cognition. Cerebellum. 2016;15:732–743.
Sayah S, Rotgé J-Y, Francisque H, et al. Personality and neuropsychological profiles in Friedreich ataxia. Cerebellum. 2018;17:204–212.
Chen D-H, Latimer C, Yagi M, et al. Heterozygous STUB1 missense variants cause ataxia, cognitive decline, and STUB1 mislocalization. Neurol Genet. 2020;6:1–13.
Acknowledgements
We are very grateful to the patients and their families for participating. Many thanks to Ludivine Chamard, Pierre Clavelou, Nathalie Daluzeau, Didier Deffond, Bernard Floccard, Pascal Labouret, Isabelle Le Ber, Roberto Marconi, Pascal Menage, Michel Obadia, Elisabeth Ollagnon Roman, Anne-Marie Ouvrard-Hernandez, Michel Rendu, Vincent de la Sayette, Xavier Soulages, Philippe Svrin, Maya Tchikvildazé, Jacques Touchon, Urielle Ullmann, Fausto Viader, Francois Viallet, and Marc Wagner, who referred cases. Many thanks to the Pitié-Salpêtrière Brain and DNA and cell banks. Members of the SPATAX network are listed in Supplementary data. Funding provided by Grand Prix Lamonica de Neurologie (Académie des Sciences).
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Disclosure
The authors declare no conflicts of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Roux, T., Barbier, M., Papin, M. et al. Clinical, neuropathological, and genetic characterization of STUB1 variants in cerebellar ataxias: a frequent cause of predominant cognitive impairment. Genet Med 22, 1851–1862 (2020). https://doi.org/10.1038/s41436-020-0899-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41436-020-0899-x
Keywords
This article is cited by
-
A Chinese Family with Digenic TBP/STUB1 Spinocerebellar Ataxia
The Cerebellum (2024)
-
Two more families supporting the existence of monogenic spinocerebellar ataxia 48
Neurogenetics (2024)
-
The inherited cerebellar ataxias: an update
Journal of Neurology (2023)
-
Multifaceted Roles of AFG3L2, a Mitochondrial ATPase in Relation to Neurological Disorders
Molecular Neurobiology (2023)
-
Spinocerebellar ataxia type 17-digenic TBP/STUB1 disease: neuropathologic features of an autopsied patient
Acta Neuropathologica Communications (2022)