Abstract
Purpose
All women diagnosed with breast cancer (BC) ≤age 50 should be referred for genetic counseling (GC) and testing. We sought to compare differences in provider practices and access across a racially and ethnically diverse population of young BC survivors.
Methods
A registry-based sample of women diagnosed with invasive BC ≤age 50 from 2009 to 2012 was recruited through the Florida Cancer Registry, and completed a questionnaire and medical record release. Differences were compared across those tested with or without the involvement of a board-certified or credentialed genetics health professional (GHP) in (1) clinical and demographic variables and (2) pretest GC elements.
Results
Of 1622 participants, there were 440 Blacks, 285 Hispanics, and 897 Non-Hispanic Whites. Of 831 participants with medical record verification of testing provider, 170 (20%) had documentation of GHP involvement. Among the 613 who recalled a pretest discussion and had GC elements collected, those with GHP involvement were significantly more likely to recall the seven recognized GC elements.
Conclusion
GHP involvement was associated with adherence to nationally recommended best practices. With the expanding importance of identifying inherited cancers, it is critical to ensure equitable access to best practices across all populations.
Similar content being viewed by others
INTRODUCTION
Breast cancer is the most common cancer among women worldwide, with 2 million new cases diagnosed in 2018.1 Hereditary breast cancer (HBC) accounts for approximately 5–10% of all breast cancer cases, most commonly due to pathogenic/likely pathogenic (P/LP) variants in the BRCA1 and BRCA2 (BRCA) genes.2 Female BRCA carriers have a 60–70% lifetime risk of developing breast cancer compared with 12% in the general population,2 and up to a 50% or greater risk of developing a second primary breast cancer.3,4,5,6,7 Evidence-based interventions such as risk-reducing surgery and breast cancer screening exist for cancer prevention and early diagnosis among BRCA carriers.8,9,10 Moreover, in addition to the personal impact of identifying HBC, this information may be shared with at-risk family members to amplify the benefits of testing and subsequent care among those at high risk.
National practice guidelines, developed and updated at least annually through the National Comprehensive Cancer Network (NCCN), recommend identification and referral for genetic counseling (GC) and consideration of testing of all women diagnosed with breast cancer at or below age 50.11 These practice guidelines also set forth recommendations for content and elements that should be discussed during the pretest GC session which may be directed by a board-certified or credentialed genetics health professional (GHP) or a health professional without formal certification in genetics (non-GHP). The NCCN guidelines are aligned with pretest GC discussion elements set forth by the National Society of Genetic Counselors (NSGC) and the American Society of Clinical Oncology (ASCO).11,12,13
The national shortage of GHPs14 coupled with particularly limited access in rural areas,15,16 certain states,17 and community oncology practices18 has resulted in most testing in the United States being performed without the inclusion of a GHP.19,20,21 Further challenges may include lack of referral to genetic counseling services, either in person or through telehealth, among providers with limited proficiency in genetics, as well as reimbursement challenges with the Centers for Medicare and Medicaid Services (CMS) not currently recognizing genetic counselors as billing providers. Consequently, there is great variability in adherence to pretest GC practices as defined by national practice guidelines. Adding to the logistical complexities of testing and in the face of GHP shortages, there are policies that mandate pretest GC be conducted with involvement of a GHP, which may disproportionately reduce testing rates among minority and underserved populations.22,23 To our knowledge, no prior population-based efforts have evaluated delivery of hereditary cancer services based on patient-reported GC elements covered by the ordering provider across a diverse population of young breast cancer survivors. Through the proposed study, we sought to compare a population-based sample of young Black, Hispanic, and Non-Hispanic White (NHW) female breast cancer survivors for differences across those tested with or without GHP involvement in (1) clinical and demographic variables and (2) content of pretest GC discussion.
MATERIALS AND METHODS
Eligible patients were women diagnosed with invasive breast cancer ≤age 50 between 2009 and 2012, living within Florida at the time of diagnosis, and alive at the time of recruitment. Information was obtained on all eligible participants from the Florida Cancer Registry (FCR) after obtaining approval from the institutional review boards at the University of South Florida and the Florida Department of Health, as previously described.24
Participants were recruited using previously described state-mandated recruitment methods,25,26 which consisted of two separate mailings sent at least three weeks apart, which included a “telephone response card” to give potential participants the option to either express interest in participation with follow-up by a study team member or decline to participate (i.e., indicate they did not wish to be contacted by phone or future mailings).27 If no response was received within three weeks of the second mailing, a member of the study team attempted to contact the potential participant by telephone to explain the study and determine interest in participation. For those willing to participate, informed consent was secured, and a baseline study questionnaire was requested.
Age at diagnosis, racial/ethnic group, and primary insurance payer at diagnosis of all potential participants were obtained from the FCR. Participants were categorized through self-reported race/ethnicity into Black, Hispanic, and NHW groups. Additional information obtained through the baseline questionnaire included college education, income, insurance status, marital status, and family history of breast and ovarian cancer.
The FCR provided the stage at diagnosis, histologic subtype, and tumor receptor status of all participants. Participants were asked to complete an authorization for release of medical records for verification of clinical information and genetic testing. Participants were further categorized into groups based on those who received GC and/or testing by a GHP. GHPs were defined as genetic counselors or medical geneticists and non-GHPs were defined as any other health-care provider, which included physicians, physician assistants, nurse practitioners, or nurses. Women who recalled having pretest GC were then asked a multipart question about whether they recalled seven pretest GC elements recommended per national guidelines,11,12,13 as listed in Table 2.
Clinical and demographic variables for all eligible participants were summarized by involvement of GHP using descriptive statistics. Using multiple logistic regression, we assessed if the following variables were associated with GHP involvement: (1) socioeconomic status (SES) (i.e., income, college education, and private insurance), (2) racial/ethnic group (Black, Hispanic, and NHW), and (3) clinical factors (i.e., age at diagnosis and family history of breast or ovarian cancer).
Women tested through a GHP were compared using Pearson chi-squared tests with those tested through a non-GHP based on (1) recall of pretest discussion and (2) seven pretest GC elements controlling the familywise type 1 error using an alpha of 0.05 and Holm step-down procedure. We also used a two-tailed, independent samples t-test to evaluate if there was a statistically significant difference in the sum of elements recalled between the GHP and non-GHP groups.
RESULTS
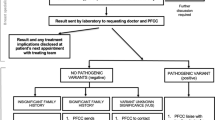
There were a total of 1622 participants, including 440 Blacks, 285 Hispanics, and 897 NHW (Fig. 1). Of these, 4 participants were excluded because GC and/or testing information was missing. Of the remaining 1618 study participants, 831 had genetic testing and provider information verified. GHP involvement (either through consultation and/or test ordering) was reported in 170 women (20%) whereas the remaining 661 women (80%) had no documentation of GHP involvement.
Of the participants that had genetic testing, there were no significant differences in GHP involvement based on participant age at diagnosis, family history of breast or ovarian cancer, college education, and annual household income ≥$50,000. Compared with NHW, Blacks and Hispanics had similar rates of GHP involvement. Based on the results of the multiple logistic regression model, private insurance was the only variable inversely associated with GHP involvement (odds ratio [OR] = 0.60, 95% confidence interval [CI]: 0.39–0.93, p = 0.02) (Table 1).
Among tested participants with GHP involvement, 84.7% recalled having a pretest discussion compared with 71.3% without GHP involvement (p < 0.001). Among the subset of 613 participants who recalled a pretest discussion and had GC elements collected, those with GHP involvement were more likely to recall specific GC elements and these differences were statistically significant for all elements analyzed, including the seven endorsed by ASCO (Table 2). In addition, the mean number of items recalled when a GHP was involved was 4.80 compared with 3.06 when a GHP was not involved. The mean difference between the GHP and non-GHP groups was statistically significant (1.74 [95% CI: 1.38–2.09]).
DISCUSSION
To our knowledge, this is among the first population-based studies to evaluate the impact of GHP involvement in the delivery and quality of pretest hereditary cancer GC and testing services across a racially and ethnically diverse population of young breast cancer survivors. Our results indicate that 20% of young breast cancer survivors are tested through a GHP. Furthermore, women with private insurance were less likely to receive care through a GHP after controlling for other clinical and socioeconomic variables. Finally, GHPs were more likely to conduct pretest GC and cover more pretest GC discussion elements based on national practice guidelines, compared with other providers without certification in genetics.
In our study, only 20% of women had genetic testing services provided by a GHP. Although survey data on patients tested for HBC by ordering provider is limited, a prior study surveyed 666 patients with early-stage breast cancer on the type of health-care provider that ordered the genetic test (surgeon, medical oncologist, primary care provider, genetic counselor, or other).28 Similar to our findings, approximately 21% of patients who received genetic testing reported that a genetic counselor ordered their genetic test.28 Demand and indications for testing have continued to rise and outpace the supply of GHPs.14,17,18 Consequently, it has become critical to develop strategies to promote best practices for delivering GC and testing services, improve proficiency in genetics, and promote partnerships to improve overall gene-based care provided to patients among non-GHPs to address several unmet needs.29
Our study is the first to evaluate the impact of socioeconomic and clinical factors on GHP involvement in a patient’s care. Prior studies to evaluate the impact of sociodemographic factors (e.g., race, age, education, and insurance status) on the receipt of GC suggest that Black women are less likely to receive GC than White women.30,31,32 It is well established that insured populations with higher SES-related indicators are overrepresented among populations who utilize GC and testing for hereditary cancer through a GHP.27,33 Furthermore, prior studies have reported that women with higher SES indicators are more likely to receive care through a GHP compared with women with public or no insurance.33,34 Yet women with private insurance in our study were more likely to receive care without the involvement of a GHP. This is surprising given hereditary cancer GC and testing through a GHP is a covered service among most private insurers, with some insurers making this a requirement in order for testing to be covered.35 It is possible that some providers refer patients to a GHP in instances where coverage may be a challenge, given that GHPs may be more familiar with coverage options for some of these patients, particularly among underinsured or uninsured populations.
In our racially, ethnically, and socioeconomically diverse population, our findings suggest that GHPs (compared with non-GHPs) were more likely to conduct pretest GC (86.5% vs. 75.7%; p < 0.001) and cover more pretest GC discussion elements based on national practice guidelines (4.80% vs. 3.06%; 1.74 [95% CI: 1.38–2.09]). These findings are consistent with our prior study comparing pretest discussion of standard pretest GC elements among a primarily NHW sample of insured patients who received BRCA testing across diverse providers and settings.24 Results from this prior study indicated that GHP involvement was associated with conduct of pretest GC, as well as with higher adherence to discussion of specific nationally recommended pretest GC elements. Pretest GC through a GHP has also been associated with increased recall about the implications of testing and the laws in place to prohibit insurance companies from denying coverage on the basis of the genetic test result.36 Consequently, with expansion of gene-based care for those at risk for inherited cancer, these gaps highlight the need to standardize the delivery of pretest GC to improve care across diverse populations and health-care settings.
The current study has several strengths including being the first registry-based study to systematically compare associations between socioeconomic and clinical factors and access to GHP among an ethnically and racially diverse sample of young breast cancer survivors treated across multiple health-care settings, enhancing the generalizability of our findings. Furthermore, our estimates of provider discussion and genetic testing across diverse populations provide updated and novel data, compared with prior efforts with more limited minority representation32,36,37 or older cohorts.31,38 Despite these strengths, there remain some limitations, including that although participants were diagnosed within the same four years and eligibility criteria were the same, the Blacks and non-Blacks were recruited under separate protocols, which could contribute to recall bias across populations. However, systemic verification of all available medical records was conducted to confirm self-reported baseline questionnaire data and minimize the impact of recall bias. It is also possible that our definition of a GHP, limited to health-care providers board certified in genetics, may have led to underestimating the magnitude of the differences between those health-care providers without expertise or additional training in genetics. This is because there are many other health-care providers without board certification in genetics who have robust expertise in this content area.
With the tremendous advances in genetic testing for use across the cancer prevention and control continuum, including importance in guiding personalized treatments,39 we expect the demand for testing to continue to rise rapidly. These factors highlight the need to develop strategies that promote robust partnerships across GHPs and non-GHPs while also supporting educational efforts to improve overall proficiency in genetics across the entire health-care workforce. This is absolutely essential to improve the provision of guideline-adherent GC services for hereditary cancer predisposition across populations, and broadly promote identification of high-risk populations and GC, as recently highlighted by the US Preventive Services Task Force (USPTSF).40 Given that most hereditary cancer GC and testing is provided by those without formal certification or training in genetics, promoting strategies for them to partner with GHPs to assist them in providing guideline-adherent service is critical.29 Standardizing the quality of genetic services provided to all racial and ethnic groups is paramount with the expanding importance of gene-based care.
In summary, this is the first population-based study across a racially and ethnically diverse population to demonstrate that GHPs were more likely to discuss GC elements, as recommended per national practice guidelines, which have potential legal, psychosocial, and cultural implications. Our findings also suggest that women with private insurance were less likely to be tested through a GHP, which is considered the gold standard and is a covered service by most private insurers. Taken together, the key differences among those in whom testing was conducted with and without the involvement of a GHP can be used to identify important implementation strategies to enhance guideline-adherent care when conducting genetic testing for HBC across diverse populations and settings.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer Clin J. 2018;68:394–424.
Felix GES, Zheng Y, Olopade OI. Mutations in context: implications of BRCA testing in diverse populations. Fam Cancer. 2018;17:471–483.
Yedjou CG, Sims JN, Miele L, et al. Health and racial disparity in breast cancer. In: Ahmad A, editor. Breast cancer metastasis and drug resistance: challenges and progress. Cham, Switzerland: Springer; 2019. p. 31–49.
Metcalfe K, Gershman S, Lynch HT, et al. Predictors of contralateral breast cancer in BRCA1 and BRCA2 mutation carriers. Br J Cancer. 2011;104:1384–1392.
Pierce LJ, Phillips K-A, Griffith KA, et al. Local therapy in BRCA1 and BRCA2 mutation carriers with operable breast cancer: comparison of breast conservation and mastectomy. Breast Cancer Res Treat. 2010;121:389–398.
Menes TS, Terry MB, Goldgar D, et al. Second primary breast cancer in BRCA1 and BRCA2 mutation carriers: 10-year cumulative incidence in the Breast Cancer Family Registry. Breast Cancer Res Treat. 2015;151:653–660.
Malone KE, Begg CB, Haile RW, et al. Population-based study of the risk of second primary contralateral breast cancer associated with carrying a mutation in BRCA1 or BRCA2. J Clin Oncol. 2010;28:2404–2410.
Salhab M, Bismohun S, Mokbel K. Risk-reducing strategies for women carrying BRCA1/2 mutations with a focus on prophylactic surgery. BMC Womens Health. 2010;10:28–28.
Warner E. Screening BRCA1 and BRCA2 mutation carriers for breast cancer. Cancers (Basel). 2018;10:477.
Carbine NE, Lostumbo L, Wallace J, Ko H. Risk-reducing mastectomy for the prevention of primary breast cancer. Cochrane Database Syst Rev. 2018;4:CD002748–CD002748.
National Comprehensive Cancer Network. Genetic/familial high-risk assessment: breast, ovarian and pancreatic. (Version1.2020). https://www-nccn-org.proxy.library.vanderbilt.edu/. Accessed 5 December 2019.
Riley BD, Culver JO, Skrzynia C, et al. Essential elements of genetic cancer risk assessment, counseling, and testing: updated recommendations of the National Society of Genetic Counselors. J Genet Couns. 2012;21:151–161.
Robson ME, Bradbury AR, Arun B, et al. American Society of Clinical Oncology policy statement update: genetic and genomic testing for cancer susceptibility. J Clin Oncol. 2015;33:3660–3667.
Penon-Portmann M, Chang J, Cheng M, Shieh JT. Genetics workforce: distribution of genetics services and challenges to health care in California. Genet Med. 2019;22:227–231.
Bookman T. More people seek genetic testing, but there aren’t enough counselors. http://www.npr.org/sections/health-shots/2016/04/18/473066953/more-people-seek-genetic-testing-but-there-arent-enough-counselors. Accessed 11 October 2016.
Bureau of Labor Statistics. Occupational employment statistics: occupational employment and wages, May 2016. 29-9092 Genetic counselors. https://www.bls.gov/oes/2016/may/oes299092.htm. Accessed 1 Nov 2019.
Villegas C, Haga SB. Access to genetic counselors in the Southern United States. J Pers Med. 2019;9:E33.
Hoskovec JM, Bennett RL, Carey ME, et al. Projecting the supply and demand for certified genetic counselors: a workforce study. J Genet Couns. 2018;27:16–20.
Acheson LS, Stange KC, Zyzanski S. Clinical genetics issues encountered by family physicians. Genet Med. 2005;7:501–508.
Vig HS, Armstrong J, Egleston BL, et al. Cancer genetic risk assessment and referral patterns in primary care. Genet Test Mol Biomarkers. 2009;13:735–741.
Radford C, Prince A, Lewis K, Pal T. Factors which impact the delivery of genetic risk assessment services focused on inherited cancer genomics: expanding the role and reach of certified genetics professionals. J Genet Couns. 2014;23:522–530.
Whitworth P, Beitsch P, Arnell C, et al. Impact of payer constraints on access to genetic testing. J Oncol Pract. 2017;13:e47–e56.
Stenehjem DD, Au T, Sainski AM, et al. Impact of a genetic counseling requirement prior to genetic testing. BMC Health Serv Res. 2018;18:165.
Cragun D, Camperlengo L, Robinson E, et al. Differences in BRCA counseling and testing practices based on ordering provider type. Genet Med. 2015;17:51–57.
Pal T, Rocchio E, Garcia A, Rivers D, Vadaparampil S. Recruitment of black women for a study of inherited breast cancer using a cancer registry–based approach. Genet Test Mol Biomarkers. 2010;15:69–77.
Bonner D, Pal T, Tallo C, Vadaparampil ST. Abstract A33: the utility of a state-wide cancer registry in recruiting a clinically representative population-based sample of young black women diagnosed with early-onset breast cancer. Cancer Epidemiol Biomarkers Prev. 2012;21 Suppl 10:A33–A33.
Cragun D, Weidner A, Lewis C, et al. Racial disparities in BRCA testing and cancer risk management across a population-based sample of young breast cancer survivors. Cancer. 2017;123:2497–2505.
Kurian AW, Li Y, Hamilton AS, et al. Gaps in incorporating germline genetic testing into treatment decision-making for early-stage breast cancer. J Clin Oncol. 2017;35:2232–2239.
Pal T, Radford C, Weidner A, Tezak AL, Cragun D, Wiesner GL. The Inherited Cancer Registry (ICARE) initiative: an academic-community partnership for patients and providers. Oncol Issues. 2018;33:54–63.
Bellcross CA, Leadbetter S, Alford SH, Peipins LA. Prevalence and healthcare actions of women in a large health system with a family history meeting the 2005 USPSTF recommendation for BRCA genetic counseling referral. Cancer Epidemiol Biomarkers Prev. 2013;22:728–735.
Hall M, Olopade OI. Confronting genetic testing disparities: knowledge is power. JAMA. 2005;293:1783–1785.
Levy DE, Byfield SD, Comstock CB, et al. Underutilization of BRCA1/2 testing to guide breast cancer treatment: Black and Hispanic women particularly at risk. Genet Med. 2011;13:349–355.
Katz SJ, Ward KC, Hamilton AS, et al. Gaps in receipt of clinically indicated genetic counseling after diagnosis of breast cancer. J Clin Oncol. 2018;36:1218–1224.
Hull LE, Haas JS, Simon SR. Provider discussions of genetic tests with U.S. women at risk for a BRCA mutation. Am J Prev Med. 2018;54:221–228.
Cigna. Genetic testing and counseling resources. 2016. https://www.cigna.com/health-care-providers/resources/genetic-testing-and-counseling-program.
Scott D, Friedman S, Telli ML, Kurian AW. Decision making about genetic testing among women with a personal and family history of breast cancer. J Oncol Pract. 2019 Oct 15; doi:10.1200/JOP.19.00221 [Epub ahead of print].
Barcenas CH, Shafaee MN, Sinha AK, et al. Genetic counseling referral rates in long-term survivors of triple-negative breast cancer. J Natl Compr Canc Netw. 2018;16:518–524.
Armstrong K, Micco E, Carney A, Stopfer J, Putt M. Racial differences in the use of BRCA1/2 testing among women with a family history of breast or ovarian cancer. JAMA. 2005;293:1729–1736.
Robson M, Im S-A, Senkus E, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017;377:523–533.
US Preventive Services Task Force. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;322:652–665.
Acknowledgements
This work was supported by grants from the Bankhead Coley granting agency (4BB15 and IBG10–34199) and the National Cancer Institute (NCI) (R01 CA204819), and in part by the Ingram Professorship, the Kleberg Foundation, and by Vanderbilt Genetic Institute departmental funds (T.P.). Support for S.R.’s time was provided by a NCI T32 training grant awarded to Vanderbilt University (T32 CA160056).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
The authors declare no conflicts of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Reid, S., Cragun, D., Tezak, A. et al. Disparities in BRCA counseling across providers in a diverse population of young breast cancer survivors. Genet Med 22, 1088–1093 (2020). https://doi.org/10.1038/s41436-020-0762-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41436-020-0762-0
Keywords
This article is cited by
-
Including multiracial individuals is crucial for race, ethnicity and ancestry frameworks in genetics and genomics
Nature Genetics (2023)
-
The ENGAGE study: evaluation of a conversational virtual agent that provides tailored pre-test genetic education to cancer patients
Journal of Cancer Survivorship (2023)
-
An overview of genetic services delivery for hereditary breast cancer
Breast Cancer Research and Treatment (2022)
-
The role of genomics in global cancer prevention
Nature Reviews Clinical Oncology (2021)