Abstract
Purpose
We sought to determine if a novel online health tool, called Down Syndrome Clinic to You (DSC2U), could improve adherence to national Down syndrome (DS) guidelines. We also sought to determine if primary care providers (PCPs) and caregivers are satisfied with this personalized online health tool.
Methods
In a national, randomized controlled trial of 230 caregivers who had children or dependents with DS without access to a DS specialist, 117 were randomized to receive DSC2U and 113 to receive usual care. The primary outcome was adherence to five health evaluations indicated by national guidelines for DS. DSC2U is completed electronically, in all mobile settings, by caregivers at home. The outputs—personalized checklists—are used during annual wellness visits with the patient’s PCP.
Results
A total of 213 participants completed a 7-month follow-up evaluation. In the intention-to-treat analysis, the intervention group had a 1.6-fold increase in the number of indicated evaluations that were recommended by the primary care provider or completed compared with controls. Both caregivers and PCPs reported high levels of satisfaction with DSC2U.
Conclusions
DSC2U improved adherence to the national DS health-care guidelines with a novel modality that was highly valued by both caregivers and PCPs.
Similar content being viewed by others
INTRODUCTION
Current estimates suggest that the population prevalence of individuals with Down syndrome (DS) in the United States is approximately 212,000 and growing, mostly due to their longer lifespan, now ~60 years.1,2 Patients with DS are prone to multiple chronic conditions and neurobiological alterations over their lifetime, many of which can be prevented and treated.3,4
In 2011, the American Academy of Pediatrics (AAP) updated its 2001 DS guidelines for pediatricians,3 and caregiver-friendly checklists have been posted online since 2013.5 Experts also developed consensus-based guidelines for internists caring for adults with DS since 2001.6,7,8 However, patients with DS are adherent to only 10–67% of these guidelines when under the care of primary care providers (PCPs) according to a few regional studies.9,10,11,12 DS-specific specialty clinics were formed to address this gap in care, and their positive impact on the diagnosis and management of co-occurring conditions is known.8 Yet, the 71 US-based DS clinics serve fewer than 5% of people with DS.13 DS clinics are geographically inaccessible for many; greater than 33% of patients with DS would need to drive more than 2 hours to reach the nearest clinic.13 As such, the majority of people with DS are likely not receiving adequate evidence-based screening and preventive care, resulting in delayed, missed, or undertreated comorbidities. To address this disparity, we created Down Syndrome Clinic to You (DSC2U), a novel, web-based tool that automatically generates personalized recommendations from symptoms and historical data entered by caregivers.
MATERIALS AND METHODS
Ethics
The research plan was approved by the Partners Human Research Committee. Informed consent was obtained from all subjects and archived at Massachusetts General Hospital.
We conducted a national two-arm, randomized controlled trial of caregivers of individuals with DS to assess the efficacy of DSC2U in assuring adherence to evidence-based guidelines (ClinicalTrials.gov: NCT04227197; HSRProj number: HSRP20163014). The full protocol is available, on request, from the corresponding author.
Participants
Participants were recruited through online social media postings from MassGeneral Hospital and DS nonprofit organizations around the United States. We enrolled US-based caregivers between 3 October 2017 and 30 September 2018. We defined “caregivers” as parents, siblings, or other persons responsible for the care of an individual with DS whom they identify as a “dependent”. To be eligible, caregivers had to be English- or Spanish-speaking, live in the United States, and have a child/dependent with DS ≥ 1 year old, and not be an active patient in a DS specialty clinic. To strive for a more demographically diverse sample, we applied a quota system during enrollment based on the race and ethnicity of the individual with DS (Supplementary Materials S1, Figure S1). Additional recruitment measures were also implemented: (1) we reached out to the minority working groups of the DS organizations (e.g., National Down Syndrome Congress and Massachusetts Down Syndrome Congress); (2) we recontacted all of the DS local support groups in demographic areas of the country that have sizable black/African American and Spanish-speaking communities; (3) we asked African American caregivers who are influencers on social media to help recruit for our study; and (4) all of our recruitment materials, online and in print, were also available in Spanish. All caregivers provided informed consent.
Intervention
DSC2U is a web-based tool, in English and Spanish, for families to get up-to-date, personalized health and wellness information, based on national guidelines and expert consensus, for a person with DS (Supplementary Materials S2 and S3). After the caregiver completed the DSC2U intake questionnaire, they received online access to a personalized caregiver checklist and PCP plan, also available in English and Spanish. These documents contained customized suggestions that were designed to help people with DS get health care tailored to their own specific needs. Also included in the documents was in-depth information about education, therapies, life skills, mental health/neurobehavioral conditions, and psychosocial supports with extensive links to curated online resources (Supplementary Materials S3, S16, S17). Caregivers were encouraged to share and discuss the PCP plan at the next wellness visit with the PCP.
Randomization and treatment groups
Caregivers completed a baseline assessment survey (Supplemental Material S4, S20) no more than 8 weeks prior to the scheduled wellness visit with the PCP. This survey collected demographic data about the participant, their child or dependent, and their PCP; current health concerns and history; and health-care screening status. After the baseline assessment was received, caregivers were assigned in a 1:1 ratio to DSC2U or waitlist according to a computer-generated randomization schedule constructed with permuted blocks of size 2 and 4, stratified for traveling distance from PCP (three levels: <30 minutes, 30–59 minutes, and ≥60 minutes) and type of insurance (two levels: public and private). We emailed participants randomized to the intervention group a link to DSC2U, accessible with a 4-digit passcode so that they could return to DSC2U and complete it at their convenience. The DSC2U intake questionnaire contains ten sections for caregivers to complete, including current symptoms in their loved one with DS along with any past medical or behavioral diagnoses and any recent blood work or diagnostic testing. The DSC2U intake also contains optional questions about nutrition, education, therapies, life skills, and community resources (Supplementary Materials S3). The DSC2U intake questionnaire is distinct from the baseline assessment.
If the DSC2U intake questionnaire was not completed, we emailed reminders at 4, 3, and 2 weeks before the scheduled PCP appointment. Participants in the control group received usual care for 7 months after their next scheduled PCP appointment, after which they were offered access to DSC2U.
Outcomes
The primary outcome was adherence to five recommended health-care evaluations: celiac screen, sleep study, thyroid test, audiogram, and ophthalmology evaluation (Supplementary Materials S2, S7).3,4,5,6 These five were selected from among those set forth by the AAP and adult consensus statements for DS because of the prevalence of the associated medical problems, the consequences of failing to treat appropriately, and the availability of treatments and therapies. We assessed the indications for each of the five evaluations for a given participant based on information reported in the baseline assessment (Supplementary Material S21). Our secondary outcomes were experience with the PCP visit, satisfaction with DSC2U (intervention group), and quality of life measures.
Data collection
Primary outcomes were assessed by caregiver survey approximately 7 months after the PCP visit to allow time for recommended evaluations to be scheduled and completed. We asked caregivers whether the five evaluations were completed or recommended by the PCP (Supplementary Material S18). Primary outcome evaluations were measured along with other health-care evaluations to assess response bias. To validate caregiver responses, medical records were obtained from the first 20 caregivers who completed the study. We planned for validation of all caregiver reports if agreement was less than 90%.
Secondary outcomes measuring the experience with the PCP visit were gathered by caregiver survey approximately 2 weeks after the PCP visit for both the intervention and control participants (Supplementary Material S6, Figures S6, S7). Some measures of visit experience were taken or adapted from the Clinician Group Consumer Assessment of Healthcare Providers and Systems (CG-CAHPS) suite of instruments (Supplementary Material S19).14 We also asked the intervention group about their satisfaction with DSC2U at the 2-week and 7-month time points.
At baseline and then ~2 weeks and ~7 months after the PCP visit, six measures of quality of life were collected by caregiver self-report using age-appropriate versions of the PedsQL 2.0 Family Impact Module and PedsQL 4.0 parent-proxy, standard Short Form 15 Generic Core Scales (Supplementary Material S5).15,16,17
PCP-reported measures, including experience with the patient visit and experience with DSC2U (intervention arm), were gathered by self-administered mail surveys sent 2 weeks after the visit (Supplementary Material S20). An incentive of $40 was enclosed with the survey.
Except where indicated otherwise, all caregiver communications, including survey requests, were by email. Reminder emails to nonrespondents were sent up to three times about two weeks apart, concluding with two phone calls in the eighth week, if needed. PCPs received their survey by mail, with an option to complete the survey electronically; reminders were sent by mail up to two times, two weeks apart, concluding with two phone calls in the sixth week, if needed. All study data were collected and managed using REDCap.18,19 Caregivers received up to $50 in incentives and an additional $20 if selected for validation.
Statistical analysis
The complete statistical analysis plan is available in Supplementary Materials S8 and S9. Based on previously published completion rates of indicated evaluations10 and to allow for up to a 14% dropout, we estimated that a sample of 200 total parents/caregivers would yield 80% power to detect an average increase of 0.6 completed or recommended evaluations. This sample would provide 80% power to detect treatment-specific improvements in secondary outcomes for effect sizes as small as 0.56. All analyses were performed on an intention-to-treat basis. All tests for significance were two-sided. Analyses were performed using SAS (version 9.4, SAS Institute, Cary, NC).
Significant benefit, defined a priori, was declared when the mean number of indicated evaluations that had been recommended or completed was greater among participants randomized to the intervention if two-sample t-test with a two-tailed p value for the treatment comparison was <0.05. For participants lost to follow-up and those reporting that they were not sure whether a given evaluation was completed or recommended by their PCP, we counted them as having not completed the evaluation. (In this intention-to-treat analysis, we treated loss to follow-up as fully informative about noncompletion.) On the other extreme, as part of our Supplementary Materials, we performed the same analyses but excluded any missing data. (In this case we treated loss to follow-up as wholly noninformative [i.e., completely at random] with respect to noncompletion). We confirmed our inference using a generalized linear model assuming beta-binomial distributed counts of recommended or completed evaluations among those indicated with parameters estimated by maximum likelihood and in a permutation test by direct randomization of assigned treatment labels.
We analyzed longitudinal changes in our secondary outcome measures using a shared-baseline repeated-measures analysis of variance (ANOVA) for each of six quality of-life outcomes derived from the two PedsQL instruments. Linear contrasts were used to test for treatment-specific improvements over time. As a sensitivity analysis, we also used simple two-group Wilcoxon rank-sum tests to compare changes from baseline to the 2-week and 7-month follow-ups individually. With six secondary outcomes, we tested each secondary outcome at ɑ = 0.008, two-tailed, to control for multiple comparisons.
We further analyzed the primary outcome for subgroup-dependent differences with linear models that included terms for subgroup membership, treatment group, and their interaction and that allowed for heteroscedastic variance by subgroup. Evidence of a subgroup-dependent difference in the efficacy of DSC2U was based on the significance of the subgroup x treatment group interaction (Supplementary Materials S13).
RESULTS
Participant flow
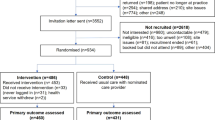
We assessed 645 caregivers for eligibility through the study website, and 281 were consented (Fig. 1). After accounting for the caregivers who did not complete the baseline survey, changed their PCP visit date outside of the study window, or self-withdrew, we randomized 230 consented caregivers to receive either DSC2U (117 participants) or “usual care” (113 participants).
aOne self-withdrew because subject lives in Canada; another self-withdrew because subject became ill and no longer had time to participate; bOne participant discontinued participation because found DS specialty clinic; another discontinued participation because uncomfortable sharing requested information with study; cOne participant did not complete PCP visit because moved states and was not able to find new PCP in study window; another did not complete PCP visit because rescheduled date outside of study window; dNon-responsive are participants who didn’t complete a survey, but then did complete a subsequent survey later in the study; eLost to follow up are participants who stop responding to surveys; fPCP of withdrawn subject was sent survey before subject withdrew; gWithdrawn subject issued 7-month follow-up survey in error and subject completed survey; hWe received surveys from some participants who had not responded at the 2-week time point.
At the 2-week follow-up, 101 (86.3%) of the 117 original caregivers in the intervention group and 108 (95.6%) of the 113 caregivers in the control group completed the surveys. At this same time point, 81.6% and 77.9% of the PCPs completed the surveys in the intervention and control groups, respectively.
At the 7-month follow-up, 103 (88.0%) of 117 original caregivers in the intervention group and 110 (97.3%) of 113 caregivers in the control group completed the surveys.
Baseline characteristics of the intervention and control groups
The ages of the individuals with DS ranged from 8 months to 56 years old (at the time of the caregivers’ completion of the baseline assessment), and 92.1% of caregivers were mothers. The two treatment groups of caregivers, patients, and PCPs were well balanced with respect to baseline characteristics and relationships (Table 1; S1, S2).
Primary outcomes
At the end of the 7-month follow-up period, the primary outcome—the number of indicated evaluations that were completed or recommended by the PCP—was greater among DSC2U participants (intervention group: M = 0.53, SD = 0.73; control group: M = 0.33, SD = 0.53; difference: 0.20, 95% confidence interval [CI]: 0.04 to 0.37, p = 0.016 by t-test, p < 0.011 by beta-binomial test, p = 0.012 by permutation test; Tables 2, 3). Participants in the intervention group had a 1.6-fold increase in the number of indicated evaluations that were completed or recommended by the PCP compared with the controls. The mean absolute difference was 0.20 evaluations across the five evaluation items between the intervention group and the control group. Put another way, the DSC2U participants had one more indicated evaluation that was recommended by their PCP or completed (versus control group) for every five PCP visits. All of these indicated evaluations were supported by the DS health-care guidelines.3,5,6,7
Of note, the intervention group was also more likely to complete nonindicated evaluations than the control group (Supplementary Materials S12) although the difference was not significant when classifying loss to follow-up as noncompletion in our intention-to-treat analysis (Tables 2, 3). None of the analyzed subgroups disproportionately benefited from DSC2U based on our primary outcome measure (Supplementary Materials S13). We validated the reports of five primary outcomes from 20 caregivers. Of these 100 parental reports, 95 were accurate based on medical records. We received five inconsistent responses from four caregivers.
Secondary outcomes
Caregivers in both arms rated the PCP visits highly (Supplementary Table S2). On a 10-point Likert scale with “10” representing “most helpful” and “0” representing “least helpful”, caregivers reported high satisfaction with DSC2U at both the 2-week follow-up (M = 7.75, SD = 1.84) and 7-month follow-up (M = 7.79, SD = 1.82); satisfaction exhibited no significant decline over this time (p = 0.90, Table 4). PCPs also expressed high satisfaction with DSC2U at the 2-week follow-up (M = 7.80, SD = 1.86).
Analyses of secondary quality of life outcomes, as ascertained through the six PedsQL 4.0 parent-proxy and PedsQL 2.0 Family Impact Module summary scores, did not demonstrate a significant difference between the intervention and control groups at either the 2-week or 7-month time points (Supplementary Material S15, Table S10).
Ancillary analyses
Of the caregivers in the intervention group who completed the 2-week follow-up (N = 101), all accessed the caregiver checklist in some capacity (viewing, downloading, and/or printing). Of these, 97% reported that the caregiver checklist recommendations were easy to understand (Table 4). All caregivers would recommend the checklist to another caregiver. About 76% would review their checklist more than twice per year after the 2-week time point, and 61% would access more than twice per year after the 7-month time point (p = 0.004, Table 4). At the 2-week time point, 96% of caregivers would recomplete DSC2U again in the future, which did not statistically lessen over time (Table 4).
When the plan was shared with the PCP, 93% of caregivers felt that the PCP seemed to be interested in the information, and 86% felt the PCPs agreed with the recommendations. At the 2-week follow-up, all PCPs who received the plan discussed the recommendations with the caregiver and were interested in the information. About 97% of PCPs who received the plan agreed with the recommendations.
None of the characteristics of the individual with DS, the caregiver, the PCP, the PCP’s practice, or the relationship between the PCP and caregiver was a statistically significant predictor for any of the dependent variables detailed above, when the p value was adjusted to account for multiple comparisons (Supplemental Material S14, Table S9).
DISCUSSION
In a national, two-arm, randomized controlled trial, patients with DS were more likely to be up to date with health-care guidelines and expert consensus if their caregivers accessed a novel health-care tool (DSC2U). This online tool directed toward the caregiver community has not only been created to support the wellness of individuals with DS, but also shown to be effective in improving health-related outcomes.
Caregivers and PCPs were highly satisfied with the tool. The majority of caregivers indicated they would revisit their caregiver checklist more than twice a year, and nearly all reported that they would recomplete DSC2U at least once a year. Nearly all PCPs agreed with the recommendations in the plans. The caregiver checklists and PCP plans also generated in-depth information about educational, neurobehavioral, psychosocial, and life skills topics, including a curated list of links to web-based resources (Supplementary Material S16 and S17). These resources may have contributed to caregivers’ and providers’ high satisfaction levels.
Our study was not without limitations. Our caregiver cohort was largely white and well educated: 73% had a 4-year college degree or higher, and only 11% scored low on our health literacy assessment (Table S1). We know that DS naturally occurs equally in all races and ethnicities, regardless of socioeconomic status. Although we were able to meet our enrollment target for Hispanics/Latinos, we were unable to achieve our targeted goal for black participants through the recruitment mechanisms described in Materials and Methods. To this extent, our results might not be generalizable to the black/African American DS community. Future research is needed to learn how to disseminate and implement DSC2U within this community.
The patients with DS represented in our study were also already very adherent to wellness guidelines at baseline: overall, 18% of patients with DS were already up to date on the five measured outcomes at baseline (Table 3b), and the remaining families were short by only 1.5 evaluations, on average (Table 3c). Their PCPs also participated with a high response rate. This level of caregiver and provider engagement might suggest that the families that enrolled in our study were actively engaged with their PCPs before the study. Yet, even within this well cared for population, a clinically significant improvement was achieved for those receiving the DSC2U intervention. We infer that for families in the United States who are less adherent to the wellness guidelines, the improvement might even be greater.
We also discovered that, when compared with controls, patients with in the intervention group were also more likely to complete nonindicated evaluations than the control group, although the difference was not significant in the intention-to-treat analysis. Receiving the PCP plan may have caused PCPs in the intervention group to pay closer attention to the care of these patients. When examined individually, two of these screenings (celiac and thyroid screening) were statistically more likely to be completed, or at least recommended, when not indicated in the intervention group. These blood tests may have been easier to obtain, or their clinical triggers might have been more likely to intersect with neurotypical health care. Sleep studies, ophthalmology exams, and hearing testing did not seem to be obtained as much when they were not clinically indicated. Notably, the caregiver checklists and PCP plans did not enumerate which evaluations were not needed; only actions on missing items were suggested. Future versions of DSC2U might consider explicitly listing evaluations that are not clinically indicated, to reduce health-care costs even further.
Our subgroup analyses—examining people with DS by race, ethnicity, language, age, and insurance type—did not demonstrate differences in our primary outcome. Further, our regression analyses did not uncover certain characteristics of the patient, caregiver, or PCP that could predict the use or relevance of DSC2U. Since this study was not powered for these secondary analyses, further research is needed to determine if effect sizes and satisfaction levels might vary among subgroups within the DS community.
DSC2U might also serve as a blueprint for caregiver and PCP engagement in improving evidence-based practice in other health-care conditions beyond DS (e.g., Williams syndrome, 22q11 syndrome, adult congenital heart disease). The adaptation of DSC2U to these conditions has the potential to buttress conversations between families and PCPs who are increasingly tasked with more complex health-care recommendations for a growing number of conditions.
Overall, DSC2U improved adherence to national DS health-care guidelines with a broadly accessible, low-touch modality that was highly appreciated by both caregivers and PCPs. This is especially relevant as in-person DS specialty clinics become both financially unsustainable and geographically limited.13 Our positive results demonstrated that our novel tool, which empowered caregivers with curated medical information, has the potential to disseminate high-cost specialty care into lower-cost primary care settings.
Change history
24 October 2020
In original version of the Article the Acknowledgement section omitted to include the contribution of DS-Connect® (The Down Syndrome Registry) which is supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), NIH, for the study recruitment used in this manuscript. This has now been added to both the PDF and HTML versions of the Article.
09 November 2020
An amendment to this paper has been published and can be accessed via a link at the top of the paper.
References
de Graaf G, Buckley F, Skotko BG. Estimation of the number of people with Down syndrome in the United States. Genet Med Off J Am Coll Med Genet. 2017;19:439–447.
de Graaf G, Buckley F, Skotko BG. Estimates of the live births, natural losses, and elective terminations with Down syndrome in the United States. Am J Med Genet A. 2015;167:756–767.
Bull MJ, Committee on Genetics Health supervision for children with Down syndrome. Pediatrics. 2011;128:393–406.
Vacca RA, Bawari S, Valenti D, et al. Down syndrome: neurobiological alterations and therapeutic targets. Neurosci Biobehav Rev. 2019;98:234–255.
American Academy of Pediatrics. Children with Down syndrome: health care information for families. September 5, 2013. https://www.healthychildren.org/English/health-issues/conditions/developmental-disabilities/Pages/Children-with-Down-Syndrome-Health-Care-Information-for-Families.aspx.
Chicoine B, McGuire D. The guide to good health for teens & adults with Down syndrome. Woodbine House; Bethesda, MD, 2010.
Steingass KJ, Chicoine B, McGuire D, Roizen NJ. Developmental disabilities grown up: Down syndrome. J Dev Behav Pediatr JDBP. 2011;32:548–558.
Smith DS. Health care management of adults with Down syndrome. Am Fam Physician. 2001;64:1031–1038.
O’Neill ME, Ryan A, Kwon S, Binns HJ. Evaluation of pediatrician adherence to the American Academy of Pediatrics health supervision guidelines for Down syndrome. Am J Intellect Dev Disabil. 2018;123:387–398.
Skotko BG, Davidson EJ, Weintraub GS. Contributions of a specialty clinic for children and adolescents with Down syndrome. Am J Med Genet A. 2013;161:430–437. https://doi.org/10.1002/ajmg.a.35795.
Santoro SL, Martin LJ, Pleatman SI, Hopkin RJ. Stakeholder buy-in and physician education improve adherence to guidelines for Down syndrome. J Pediatr. 2016;171:262–.e1.
Santoro SL, Bartman T, Cua CL, Lemle S, Skotko BG. Use of electronic health record integration for Down syndrome guidelines. Pediatrics. 2018;142:e20174119.
Joslyn N, Berger H, Skotko BG. Geospatial analyses of accessibility to Down syndrome specialty care. J Pediatr.2020;218:146–150.
Agency for Healthcare Research and Quality. About CAHPS. 2019. https://www.ahrq.gov/cahps/about-cahps/index.html. Accessed 12 December 2019.
Varni JW, Seid M, Rode CA. The PedsQLTM: measurement model for the Pediatric Quality of Life Inventory. Med Care. 1999;37:126–139.
Varni JW, Seid M, Kurtin PS. PedsQLTM 4.0: reliability and validity of the Pediatric Quality of Life InventoryTM Version 4.0 Generic Core Scales in Healthy and Patient Populations. Med Care. 2001;39:800–812.
Varni JW, Sherman SA, Burwinkle TM, Dickinson PE, Dixon P. The PedsQL Family Impact Module: preliminary reliability and validity. Health Qual Life Outcomes. 2004;2:55.
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)-a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381.
Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208.
Acknowledgements
Our research team gives special thanks to Sujata Bardhan and Melissa Parisi of the Eunice Kennedy Shriver National Institute of Child Health and Human Development for thoughtful consultation throughout this research. We are also grateful to Caitlin Woglom for providing insight into DSC2U’s nutrition-based questions and to Maggie Balz for providing insight into DSC2U’s swallowing-based questions. We are grateful to Beth Watters, Rosemary Guiltinan, and Elizabeth Azano of Partners Office of General Counsel for their legal consultation on our description of DSC2U and the customized outputs. Ye Chin Lee of the MGH Laboratory of Computer Science provided valuable advice on the long-term sustainability of DSC2U. We especially thank all of the Down syndrome nonprofit organizations that advertised our trial to their members. This work was supported through a Patient-Centered Outcomes Research Institute (PCORI) Research Award (AD-1507-31567). The authors acknowledge the contribution of DS-Connect® (The Down Syndrome Registry) which is supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), NIH, for the study recruitment used in this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
B.G.S. occasionally consults on the topic of Down syndrome through Gerson Lehrman Group. He receives remuneration from Down syndrome nonprofit organizations for speaking engagements and associated travel expenses. B.G.S. receives annual royalties from Woodbine House, Inc., for the publication of his book, Fasten Your Seatbelt: A Crash Course on Down Syndrome for Brothers and Sisters. Within the past two years, he has received research funding from F. Hoffmann-La Roche, Inc. and LuMind Research Down Syndrome Foundation to conduct clinical trials for people with Down syndrome. B.G.S. is occasionally asked to serve as an expert witness for legal cases where Down syndrome is discussed. B.G.S. serves in a nonpaid capacity on the Honorary Board of Directors for the Massachusetts Down Syndrome Congress and the Professional Advisory Committee for the National Center for Prenatal and Postnatal Down Syndrome Resources. B.G.S. has a sister with Down syndrome. K.D. is an unpaid member of the Board of Directors of Bridges Associates, a private not-for-profit organization serving children and adults with learning disabilities in Barnstable County, Massachusetts. E.A.M. serves as a DSMB member for Novartis Pharmaceuticals and Shire Human Genetic Therapies; serves on a trial Steering Committee for Biogen; serves on the Executive Committee of the Parkinson Study Group; has recently consulted for Cerevance, InTrance, and Inventram; and his institution receives research funding on his behalf from ALS Finding A Cure, Amylyx Pharmaceuticals, Autism Speaks, Cedars-Sinai Research Institute, Farmer Family Foundation, GlaxoSmithKline, Mitsubishi Tanabe Pharmaceuticals, and the Salah Foundation. S.L.S. receives research funding from the LuMind Down Syndrome Foundation IDSC. She is a nonpaid volunteer for the Medical and Scientific Advisory Council for the Massachusetts Down Syndrome Congress. S.B. is an employee of the Down Syndrome Association of Los Angeles and serves in a nonpaid capacity on the Self Determination Advisory Committee as a parent of an individual with disabilities for the North Los Angeles County Regional Center area. P.E.B. serves in a nonpaid capacity on the board of trustees of the Riverview School in East Sandwich, Massachusetts. P.E.B. has a daughter with Down syndrome. B.C. receives remuneration from Down syndrome nonprofit organizations for speaking engagements and associated travel expenses. B.C. receives annual royalties from Woodbine House, Inc., for the publication of his books, Mental Wellness in Adults with Down Syndrome and The Guide to Good Health for Teens and Adults with Down Syndrome. Within the past year, he has received research funding from LuMind Research Down Syndrome Foundation to conduct a clinical trial for people with Down syndrome. B.C. is occasionally asked to serve as an expert witness for legal cases where Down syndrome is discussed. B.C. serves in a nonpaid capacity on the Board of Directors for the Down Syndrome Medical Interest Group and the Professional Advisory Committee for the National Down Syndrome Society and the National Down Syndrome Congress. B.C.’s great uncle had Down syndrome. J.M. speaks at conferences for Down syndrome and other disability organizations. Additionally, J.M. consults and facilitates the National Down Syndrome Congress Advocacy Training Boot Camp and does some other advocacy related consulting for them, and facilitates educational workshops for a local nonprofit, Inclusion Connections. J.M. currently serves on two boards, Disability Rights Center of Kansas (incoming Board President) and the City of Olathe Persons with Disabilities Advisory Board. J.M. has a child with Down syndrome. T.R. is a self-employed pediatrician. He also provides contract work for St. John’s Health in Jackson, Wyoming as a staff pediatrician and for the State of Wyoming and Teton County, Wyoming as County Health Officer. He is a volunteer clinical faculty member of the University of Washington School of Medicine. M.S., L.M., P.E.B., S.B., J.M., and S.C. each have a child with Down syndrome. The other authors declare no conflicts of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Chung, J., Donelan, K., Macklin, E.A. et al. A randomized controlled trial of an online health tool about Down syndrome. Genet Med 23, 163–173 (2021). https://doi.org/10.1038/s41436-020-00952-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41436-020-00952-7
Keywords
This article is cited by
-
Awareness and agreement with neurofibromatosis care guidelines among U.S. neurofibromatosis specialists
Orphanet Journal of Rare Diseases (2022)