Abstract
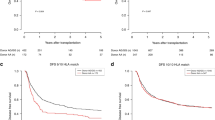
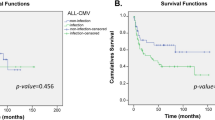
Polymorphisms in the granulocyte colony-stimulating factor receptor gene (GCSFR, CSF3R) have been reported to be associated with peripheral blood stem cell enrichment and hematological diseases. The aim of our study was to investigate the effects of donor CSF3R allelic polymorphisms on the outcomes of allogeneic stem cell transplantation. A total of 273 patients who were diagnosed with hematological diseases and treated with allogeneic hematopoietic stem cell transplantation(allo-HSCT) were enrolled in this study. Single-nucleotide polymorphisms in CSF3R were genotyped by targeted next-generation sequencing. There were six types of CSF3R genotypes with percentages over 1%. LFS and OS analyses showed that recipients receiving grafts from healthy donors with a rs3917980 G/G or A/G genotype had higher LFS rates than those receiving grafts from donors carrying a rs22754272 T/C genotype and the double-negative group (p = 0.036). Univariate cox analysis showed that donor CSF3R with the rs2275472 T/C genotype was associated with higher transplantation-related mortality (TRM) rates (HR = 2.853, 95% CI: 1.405–5.792, p = 0.00371) and lower rates of leukemia-free survival (LFS) (HR = 1.846; 95% CI: 1.018–3.347, p = 0.0435). In addition, donor CSF3R with the rs3917980G/G or A/G genotype was associated with better overall survival (OS) rates (HR = 0.560, 95% CI: 0.3162–0.9916, p = 0.047) and lower TRM rates (HR = 0.497, 95% CI: 0.2628–0.9397, p = 0.0315). Furthermore, multivariate cox analysis found that rs2275472 T/C genotype was an independent risk factors for TRM rates (HR = 3.210, 95% CI: 1.573–6.55, p = 0.001), while no statistical difference was found between rs3917980G/G or A/G genotype and clinical outcomes. Our findings demonstrate the important prognostic value of genetic variations in donor CSF3R to predict clinical outcomes in patients undergoing allo-HSCT.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 digital issues and online access to articles
$119.00 per year
only $19.83 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
References
Alvarez P, Carrillo E, Vélez C, Hita-Contreras F, Martínez-Amat A, Rodríguez-Serrano F, et al. Regulatory systems in bone marrow for hematopoietic stem/progenitor cells mobilization and homing. Biomed Res Int.2013;2013:6–8.
Pelus LM. Peripheral blood stem cell mobilization: new regimens, new cells, where do we stand. Curr Opin Hematol. 2008;15:285–92.
Horan T, Wen J, Narhi L, Parker V, Garcia A, Arakawa T, et al. Dimerization of the extracellular domain of granuloycte-colony stimulating factor receptor by ligand binding: a monovalent ligand induces 2:2 complexes. Biochemistry. 1996;35:4886–96.
Futami M, Zhu QS, Whichard ZL, Ling X, Ke YH, Corey SJ, et al. G-CSF receptor activation of the Src kinase Lyn is mediated by Gab2 recruitment of the Shp2 phosphatase. Blood. 2011;118:1077–86.
Lantow M, Sivakumar R, Zeumer L, Wasserfall C, Zheng Y, Atkinson M, et al. The granulocyte colony stimulating factor pathway regulates autoantibody production in a murine induced model of systemic lupus erythematosus. Arthritis Res Ther. 2013;15:1–14.
Mehta HM, Futami M, Glaubach T, Lee DW, Andolina JR, Yang Q, et al. Alternatively spliced, truncated GCSF receptor promotes leukemogenic properties and sensitivity to JAK inhibition. Leukemia. 2014;28:1041–51.
Hunter MG, Avalos BR. Deletion of a critical internalization domain in the CSF3R in acute myelogenous leukemia preceded by severe congenital neutropenia. Blood. 1999;93:440–6.
Ward AC, Van Aesch YM, Schelen AM, Touw IP. Defective internalization and sustained activation of truncated granulocyte colony-stimulating factor receptor found in severe congenital neutropenia/acute myeloid leukemia. Blood. 1999;93:447–58.
Boguniakubik K, Gieryng A, Gebura K, Lange A. Genetic variant of the G-CSF receptor gene is associated with lower mobilization potential and slower recovery of granulocytes after transplantation of autologous peripheral blood progenitor cells. Cytokine. 2012;60:463–7.
Camurdanoglu BZ, Esendagli G, Ozdemir E, Canpinar H, Guc D, Kansu E. The effect of granulocyte colony stimulating factor receptor gene missense single nucleotide polymorphisms on peripheral blood stem cell enrichment. Cytokine. 2013;61:572–7.
Dwivedi P, Greis KD. Granulocyte colony-stimulating factor receptor signaling in severe congenital neutropenia, chronic neutrophilic leukemia, and related malignancies. Exp Hematol. 2017;46:9–20.
Wang H, Feng L, Niu D. Relationship between mRNA stability and intron presence. Biochem Biophys Res Commun. 2007;354:203–8.
Yu A, Kimura SI, Gomyo A, Jin H, Tamaki M, Harada N, et al. Delayed platelet recovery after allogeneic hematopoietic stem cell transplantation: Association with chronic graft-versus-host disease and survival outcome. Hematol Oncol. 2018;36:276–84.
Dominietto A, Raiola AM, Lint M, Lamparelli T, Bacigalupo A. Factors influencing haematological recovery after allogeneic haemopoietic stem cell transplants : graft-versus-host disease, donor type, cytomegalovirus infections and cell dose. Br J Haematol. 2001;112:219–27.
Hisham M, Hanafy H, Amr A, Omneya H, Leslie L, Alaa H. Day+100 Platelet Count Predicts Survival After Allogeneic Hematopoietic Stem-Cell Transplantation in Children With Hematologic Malignancies. Clin Lymphoma Myeloma Leuk. 2019;19:E221–E227.
Pulanic D, Lozier JN, Pavletic SZ. Thrombocytopenia and hemostatic disorders in chronic graft versus host disease. Bone Marrow Transplant. 2009;44:393–403.
Su L, Gao SJ, Tan YH, Lin H, Liu XL, Liu SS, et al. CSF3R mutations were associated with an unfavorable prognosis in patients with acute myeloid leukemia with CEBPA double mutations. Ann Hematol. 2019;98:1641–6.
Fleischman AG, Maxson JE, Luty SB, Luty SB, Agarwal A, Royer LR, et al. The CSF3R T618I mutation causes a lethal neutrophilic neoplasia in mice that is responsive to therapeutic JAK inhibition. Blood. 2013;122:3628–31.
Funding
This study was supported by grants from the National Natural Science Foundation of China (Grant Nos. 82070184, 81870140, 81870137), the National Key Research and Development Program of China (Nos. 2017YFA0104500, 2021YFC2500300); Scientific Research Foundation for Capital Medicine Development (No. 2018-2-4084) Beijing Municipal Science & Technology Commission (No. Z171100001017098). The authors thank the members of the core facilities at the Peking University Institute of Hematology for the sample collection.
Author information
Authors and Affiliations
Contributions
XZ and X-SZ designed the study; XC performed data analysis; XC, YH, XY, LX, XZ, YW, KL, XH, and YC participated in the information collection of donors and patients. XC drafted the manuscript. All authors read and approved the final version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cao, XH., Hong, Y., Yu, X. et al. Donor CSF3R with the rs3917980A/G or G/G genotype is correlated with better leukemia-free survival after allogenic hematopoietic stem cell transplantation. Genes Immun 23, 166–174 (2022). https://doi.org/10.1038/s41435-022-00177-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41435-022-00177-5