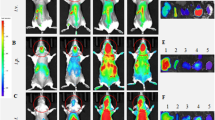
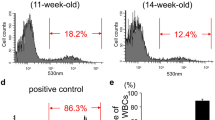
Abstract
Glial cell line-derived neurotrophic factor (GDNF) protects dopaminergic neurons in various models of Parkinson’s disease (PD). Cell-based GDNF gene delivery mitigates neurodegeneration and improves both motor and non-motor functions in PD mice. As PD is a chronic condition, this study aims to investigate the long-lasting benefits of hematopoietic stem cell (HSC)-based macrophage/microglia-mediated CNS GDNF (MMC-GDNF) delivery in an MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) mouse model. The results indicate that GDNF treatment effectively ameliorated MPTP-induced motor deficits for up to 12 months, which coincided with the protection of nigral dopaminergic neurons and their striatal terminals. Also, the HSC-derived macrophages/microglia were recruited selectively to the neurodegenerative areas of the substantia nigra. The therapeutic benefits appear to involve two mechanisms: (1) macrophage/microglia release of GDNF-containing exosomes, which are transferred to target neurons, and (2) direct release of GDNF by macrophage/microglia, which diffuses to target neurons. Furthermore, the study found that plasma GDNF levels were significantly increased from baseline and remained stable over time, potentially serving as a convenient biomarker for future clinical trials. Notably, no weight loss, altered food intake, cerebellar pathology, or other adverse effects were observed. Overall, this study provides compelling evidence for the long-term therapeutic efficacy and safety of HSC-based MMC-GDNF delivery in the treatment of PD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
Data availability
The datasets generated and/or analyzed in the current study are accessible upon reasonable request from either the first author or the corresponding author.
References
Aarsland D, et al. Parkinson disease-associated cognitive impairment. Nat Rev Dis Prim. 2021;7:47.
Dauer W, Przedborski S. Parkinson’s disease: mechanisms and models. Neuron. 2003;39:889–909.
Ahlskog JE, Muenter MD. Frequency of levodopa-related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov Disord. 2001;16:448–58.
Liu B, et al. Effects of Eldepryl on glial cell proliferation and activation in the substantia nigra and striatum in a rat model of Parkinson’s disease. Neurol Res. 2017;39:459–67.
Henchcliffe C, Severt WL. Disease modification in Parkinson’s disease. Drugs Aging. 2011;28:605–15.
Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260:1130–2.
Tomac A, et al. Protection and repair of the nigrostriatal dopaminergic system by GDNF in vivo. Nature. 1995;373:335–9.
Henderson CE, et al. GDNF: a potent survival factor for motoneurons present in peripheral nerve and muscle. Science. 1994;266:1062–4.
Kramer ER, Liss B. GDNF-Ret signaling in midbrain dopaminergic neurons and its implication for Parkinson disease. FEBS Lett. 2015;589:3760–72.
Emborg ME, et al. Response of aged parkinsonian monkeys to in vivo gene transfer of GDNF. Neurobiol Dis. 2009;36:303–11.
Kells AP, et al. Regeneration of the MPTP-lesioned dopaminergic system after convection-enhanced delivery of AAV2-GDNF. J Neurosci. 2010;30:9567–9577.
Migliore MM, et al. Neurotrophic and neuroprotective efficacy of intranasal GDNF in a rat model of Parkinson’s disease. Neuroscience. 2014;274:11–23.
Manfredsson FP, et al. The future of GDNF in Parkinson’s disease. Front Aging Neurosci. 2020;12:593572.
Tereshchenko J, Maddalena A, Bahr M, Kugler S. Pharmacologically controlled, discontinuous GDNF gene therapy restores motor function in a rat model of Parkinson’s disease. Neurobiol Dis. 2014;65:35–42.
Gill SS, et al. Direct brain infusion of glial cell line-derived neurotrophic factor in Parkinson disease. Nat Med. 2003;9:589–95.
Love S, et al. Glial cell line-derived neurotrophic factor induces neuronal sprouting in human brain. Nat Med. 2005;11:703–4.
Patel NK, et al. Intraputamenal infusion of glial cell line-derived neurotrophic factor in PD: a two-year outcome study. Ann Neurol. 2005;57:298–302.
Slevin JT, et al. Improvement of bilateral motor functions in patients with Parkinson disease through the unilateral intraputaminal infusion of glial cell line-derived neurotrophic factor. J Neurosurg. 2005;102:216–22.
Kordower JH, et al. Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson’s disease. Science. 2000;290:767–73.
Georgievska B, Kirik D, Rosenblad C, Lundberg C, Bjorklund A. Neuroprotection in the rat Parkinson model by intrastriatal GDNF gene transfer using a lentiviral vector. Neuroreport. 2002;13:75–82.
Bauer M, et al. Lipid-mediated glial cell line-derived neurotrophic factor gene transfer to cultured porcine ventral mesencephalic tissue. Exp Neurol. 2002;177:40–9.
Chtarto A, et al. Controlled delivery of glial cell line-derived neurotrophic factor by a single tetracycline-inducible AAV vector. Exp Neurol. 2007;204:387–99.
Wang L, et al. Delayed delivery of AAV-GDNF prevents nigral neurodegeneration and promotes functional recovery in a rat model of Parkinson’s disease. Gene Ther. 2002;9:381–9.
McGrath J, et al. Adeno-associated viral delivery of GDNF promotes recovery of dopaminergic phenotype following a unilateral 6-hydroxydopamine lesion. Cell Transpl. 2002;11:215–27.
Brundin P. GDNF treatment in Parkinson’s disease: time for controlled clinical trials? Brain. 2002;125:2149–51.
Sherer TB, Fiske BK, Svendsen CN, Lang AE, Langston JW. Crossroads in GDNF therapy for Parkinson’s disease. Mov Disord. 2006;21:136–41.
Salvatore MF, et al. Point source concentration of GDNF may explain failure of phase II clinical trial. Exp Neurol. 2006;202:497–505.
Kambey PA, et al. Failure of glial cell-line derived neurotrophic factor (GDNF) in clinical trials orchestrated by reduced NR4A2 (NURR1) transcription factor in Parkinson’s disease. A systematic review. Front Aging Neurosci. 2021;13:645583.
Barker RA, et al. GDNF and Parkinson’s disease: where next? a summary from a recent workshop. J Parkinsons Dis. 2020;10:875–91.
Rosenblad C, Georgievska B, Kirik D. Long-term striatal overexpression of GDNF selectively downregulates tyrosine hydroxylase in the intact nigrostriatal dopamine system. Eur J Neurosci. 2003;17:260–70.
Georgievska B, Kirik D, Bjorklund A. Overexpression of glial cell line-derived neurotrophic factor using a lentiviral vector induces time- and dose-dependent downregulation of tyrosine hydroxylase in the intact nigrostriatal dopamine system. J Neurosci. 2004;24:6437–45.
Barroso-Chinea P, et al. Long-term controlled GDNF over-expression reduces dopamine transporter activity without affecting tyrosine hydroxylase expression in the rat mesostriatal system. Neurobiol Dis. 2016;88:44–54.
Mesa-Infante V, Afonso-Oramas D, Salas-Hernandez J, Rodriguez-Nunez J, Barroso-Chinea P. Long-term exposure to GDNF induces dephosphorylation of Ret, AKT, and ERK1/2, and is ineffective at protecting midbrain dopaminergic neurons in cellular models of Parkinson’s disease. Mol Cell Neurosci. 2022;118:103684.
Lang AE, et al. Randomized controlled trial of intraputamenal glial cell line-derived neurotrophic factor infusion in Parkinson disease. Ann Neurol. 2006;59:459–66.
Luz M, Mohr E, Fibiger HC. GDNF-induced cerebellar toxicity: a brief review. Neurotoxicology. 2016;52:46–56.
Hovland DN Jr., et al. Six-month continuous intraputamenal infusion toxicity study of recombinant methionyl human glial cell line-derived neurotrophic factor (r-metHuGDNF) in rhesus monkeys. Toxicol Pathol. 2007;35:676–92.
Hoane MR, et al. Differential in vivo effects of neurturin and glial cell-line-derived neurotrophic factor. Exp Neurol. 1999;160:235–43.
Su X, et al. Safety evaluation of AAV2-GDNF gene transfer into the dopaminergic nigrostriatal pathway in aged and parkinsonian rhesus monkeys. Hum Gene Ther. 2009;20:1627–40.
Chen C, et al. Non-toxic HSC transplantation-based macrophage/microglia-mediated GDNF delivery for Parkinson’s disease. Mol Ther Methods Clin Dev. 2020;17:83–98.
He W, et al. Development of a synthetic promoter for macrophage gene therapy. Hum Gene Ther. 2006;17:949–59.
Biju K, et al. Macrophage-mediated GDNF delivery protects against dopaminergic neurodegeneration: a therapeutic strategy for Parkinson’s disease. Mol Ther. 2010;18:1536–44.
Zhao Y, et al. GDNF-transfected macrophages produce potent neuroprotective effects in Parkinson’s disease mouse model. PLoS One. 2014;9:e106867.
Yokoi A, Ochiya T. Exosomes and extracellular vesicles: rethinking the essential values in cancer biology. Semin Cancer Biol. 2021;74:79–91.
Biju KC, et al. Bone marrow-derived microglia-based neurturin delivery protects against dopaminergic neurodegeneration in a mouse model of Parkinson’s disease. Neurosci Lett. 2013;535:24–9.
Chen C, et al. GDNF-expressing macrophages mitigate loss of dopamine neurons and improve Parkinsonian symptoms in MitoPark mice. Sci Rep. 2018;8:5460.
Ge G, et al. Regulatable lentiviral hematopoietic stem cell gene therapy in a mouse model of Parkinson’s disease. Stem Cells Dev. 2018;27:995–1005.
Sterky FH, et al. Glial cell line-derived neurotrophic factor partially ameliorates motor symptoms without slowing neurodegeneration in mice with respiratory chain-deficient dopamine neurons. Cell Transpl. 2013;22:1529–39.
Zhao Y, et al. GDNF-expressing macrophages restore motor functions at a severe late-stage, and produce long-term neuroprotective effects at an early-stage of Parkinson’s disease in transgenic Parkin Q311X(A) mice. J Control Release. 2019;315:139–49.
Choi-Lundberg DL, et al. Dopaminergic neurons protected from degeneration by GDNF gene therapy. Science. 1997;275:838–41.
Connor B, et al. Glial cell line-derived neurotrophic factor (GDNF) gene delivery protects dopaminergic terminals from degeneration. Exp Neurol. 2001;169:83–95.
Manfredsson FP, et al. Tight Long-term dynamic doxycycline responsive nigrostriatal GDNF using a single rAAV vector. Mol Ther. 2009;17:1857–67.
Hudson J, et al. Glial cell line-derived neurotrophic factor augments midbrain dopaminergic circuits in vivo. Brain Res Bull. 1995;36:425–32.
Chu Y, Kordower JH. Post-mortem studies of neurturin gene therapy for Parkinson’s disease: two subjects with 10 years CERE120 delivery. Mov Disord. 2023;38:1728–36.
Mueller KL, Hines PJ, Travis J. Neuroimmunology. Science. 2016;353:760–1.
Cartier N, et al. Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science. 2009;326:818–23.
Jackson-Lewis V, Przedborski S. Protocol for the MPTP mouse model of Parkinson’s disease. Nat Protoc. 2007;2:141–51.
Huang D, et al. Long-term changes in the nigrostriatal pathway in the MPTP mouse model of Parkinson’s disease. Neuroscience. 2018;369:303–13.
Kanter J, et al. Biologic and clinical efficacy of LentiGlobin for sickle cell disease. N Engl J Med. 2022;386:617–28.
Hayashi T, et al. Evaluation of systemic markers of inflammation in atomic-bomb survivors with special reference to radiation and age effects. FASEB J. 2012;26:4765–73.
Paix A, et al. Total body irradiation in allogeneic bone marrow transplantation conditioning regimens: a review. Crit Rev Oncol Hematol. 2018;123:138–48.
Sun L, et al. Dose-dependent decrease in anti-oxidant capacity of whole blood after irradiation: a novel potential marker for biodosimetry. Sci Rep. 2018;8:7425.
Sun L, et al. Total body irradiation causes a chronic decrease in antioxidant levels. Sci Rep. 2021;11:6716.
Acknowledgements
Images were generated in the Core Optical Imaging Facility which is supported by UT Health San Antonio and NIH-NCI P30 CA54174. Flow cytometry data were generated in the Flow Cytometry Shared Resource at UT Health San Antonio which is supported by a grant from the National Cancer Institute (P30CA054174) to the Mays Cancer Center, a grant from the Cancer Prevention and Research Institute of Texas (CPRIT) (RP210126), a grant from the National Institutes of Health (S10OD030432), and support from the Office of the Vice President for Research at UT Health San Antonio.
Funding
This study was supported by a Merit Review grant from the Department of Veterans Affairs Biomedical Laboratory Research & Development (5I01BX000737) and the Perry & Ruby Stevens Parkinson’s Disease Center of Excellence. Partial support was also provided by the Natural Science Foundation Project of Guizhou Provincial Science and Technology Department (Qiankehe Foundation-ZK 2024 General 182).
Author information
Authors and Affiliations
Contributions
Conceptualization: G.G. and S.L.; Investigation: G.G., B.P.S., B.D.G., S.Z. and Q.Z.; Formal Analysis: G.G., B.P.S., B.D.G., S.Z., Q.Z., G.H., J.C.O., R.A.C. and S.L.; Writing—Original Draft Preparation, G.G., B.P.S., B.D.G. and S.L.; Writing—Review & Editing, G.G., G.H., J.C.O., R.A.C. and S.L.; Supervision: S.L. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This study was approved by the IACUC of the University of Texas Health Science Center at San Antonio (protocol 20140100AR).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ge, G., Sivasubramanian, B.P., Geng, B.D. et al. Long-term benefits of hematopoietic stem cell-based macrophage/microglia delivery of GDNF to the CNS in a mouse model of Parkinson’s disease. Gene Ther (2024). https://doi.org/10.1038/s41434-024-00451-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41434-024-00451-3