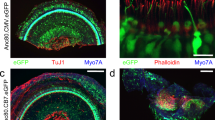
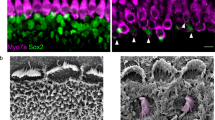
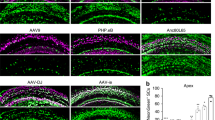
Abstract
The adeno-associated virus (AAV) gene therapy has been widely applied to mouse models for deafness. But, AAVs could transduce non-targeted organs after inner ear delivery due to their low cell-type specificity. This study compares transgene expression and biodistribution of AAV1, AAV2, Anc80L65, AAV9, AAV-PHP.B, and AAV-PHP.eB after round window membrane (RWM) injection in neonatal mice. The highest virus concentration was detected in the injected cochlea. AAV2, Anc80L65, AAV9, AAV-PHP.B, and AAV-PHP.eB transduced both inner hair cells (IHCs) and outer hair cells (OHCs) with high efficiency, while AAV1 transduced IHCs with high efficiency but OHCs with low efficiency. All AAV subtypes finitely transduced contralateral inner ear, brain, heart, and liver compared with the injected cochlea. In most brain regions, the enhanced green fluorescent protein (eGFP) expression of AAV1 and AAV2 was lower than that of other four subtypes. We suggested the cochlear aqueduct might be one of routes for vectors instantaneously infiltrating into the brain from the cochlea through a dye tracking test. In summary, our results provide available data for further investigating the biodistribution of vectors through local inner ear injection and afford a reference for selecting AAV serotypes for gene therapy toward deafness.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding authors upon reasonable request.
References
World Health Organization. Deafness and Hearing Loss. 2023. https://www.who.int/news-room/fact-sheets/detail/deafness-and-hearing-loss.
Andres-Mateos E, Landegger LD, Unzu C, Phillips J, Lin BM, Dewyer NA, et al. Choice of vector and surgical approach enables efficient cochlear gene transfer in nonhuman primate. Nat Commun. 2022; https://doi.org/10.1038/s41467-022-28969-3.
Tao Y, Liu X, Yang L, Chu C, Tan F, Yu Z, et al. AAV-ie-K558R mediated cochlear gene therapy and hair cell regeneration. Signal Transduct Target Ther. 2022; https://doi.org/10.1038/s41392-022-00938-8.
Mendes BB, Conniot J, Avital A, Yao D, Jiang X, Zhou X, et al. Nanodelivery of nucleic acids. Nat Rev Methods Primers. 2022; https://doi.org/10.1038/s43586-022-00104-y.
Rubanyi GM. The future of human gene therapy. Mol Aspects Med. 2001;22:113–42.
Botto C, Dalkara D, El-Amraoui A. Progress in gene editing tools and their potential for correcting mutations underlying hearing and vision loss. Front Genome Ed. 2021; https://doi.org/10.3389/fgeed.2021.737632.
Snow JB Jr, Wackym PA. Ballenger’s otorhinolaryngology head and neck surgery 17th. Connecticut: People’s Medical Publishing House; 2009.
Peters CW, Maguire CA, Hanlon KS. Delivering AAV to the central nervous and sensory systems. Trends Pharmacol Sci. 2021;42:461–74.
Shu Y, Tao Y, Wang Z, Tang Y, Li H, Dai P, et al. Identification of adeno-associated viral vectors that target neonatal and adult Mammalian inner ear cell subtypes. Hum Gene Ther. 2016;27:687–99.
Isgrig K, McDougald DS, Zhu J, Wang HJ, Bennett J, Chien WW. AAV2.7m8 is a powerful viral vector for inner ear gene therapy. Nat Commun. 2019; https://doi.org/10.1038/s41467-018-08243-1.
Tan F, Chu C, Qi J, Li W, You D, Li K, et al. AAV-ie enables safe and efficient gene transfer to inner ear cells. Nat Commun. 2019; https://doi.org/10.1038/s41467-019-11687-8.
Omichi R, Yoshimura H, Shibata SB, Vandenberghe LH, Smith RJH. Hair cell transduction efficiency of single- and dual-AAV serotypes in adult murine Cochleae. Mol Ther Methods Clin Dev. 2020;17:1167–77.
Landegger LD, Pan B, Askew C, Wassmer SJ, Gluck SD, Galvin A, et al. A synthetic AAV vector enables safe and efficient gene transfer to the mammalian inner ear. Nat Biotechnol. 2017; https://doi.org/10.1038/nbt.3781.
Akil O, Dyka F, Calvet C, Emptoz A, Lahlou G, Nouaille S, et al. Dual AAV-mediated gene therapy restores hearing in a DFNB9 mouse model. Proc Natl Acad Sci USA. 2019;116:4496–501.
Nist-Lund CA, Pan B, Patterson A, Asai Y, Chen T, Zhou W, et al. Improved TMC1 gene therapy restores hearing and balance in mice with genetic inner ear disorders. Nat Commun. 2019; https://doi.org/10.1038/s41467-018-08264-w.
Pan B, Askew C, Galvin A, Heman-Ackah S, Asai Y, Indzhykulian AA, et al. Gene therapy restores auditory and vestibular function in a mouse model of Usher syndrome Type 1c. Nat Biotechnol. 2017;35:264–72. https://doi.org/10.1038/nbt.3801.
Gao X, Tao Y, Lamas V, Huang M, Yeh WH, Pan B, et al. Treatment of autosomal dominant hearing loss by in vivo delivery of genome editing agents. Nature. 2018;553:217–21.
Taiber S, Cohen R, Yizhar-Barnea O, Sprinzak D, Holt JR, Avraham KB Neonatal AAV gene therapy rescues hearing in a mouse model of SYNE4 deafness. EMBO Mol Med. 2021; https://doi.org/10.15252/emmm.202013259.
Gu X, Wang D, Xu Z, Wang J, Guo L, Chai R, et al. Prevention of acquired sensorineural hearing loss in mice by in vivo Htra2 gene editing. Genome Biol. 2021; https://doi.org/10.1186/s13059-021-02311-4.
Xue Y, Hu X, Wang D, Li D, Li Y, Wang F, et al. Gene editing in a Myo6 semi-dominant mouse model rescues auditory function. Mol Ther. 2022; https://doi.org/10.1016/j.ymthe.2021.06.015.
Zheng Z, Li G, Cui C, Wang F, Wang X, Xu Z, et al. Preventing autosomal-dominant hearing loss in Bth mice with CRISPR/CasRx-based RNA editing. Signal Transduct Target Ther. 2022; https://doi.org/10.1038/s41392-022-00893-4.
Mathiesen SN, Lock JL, Schoderboeck L, Abraham WC, Hughes SM. CNS transduction benefits of AAV-PHP.eB over AAV9 are dependent on administration route and mouse strain. Mol Ther Methods Clin Dev. 2020;19:447–58.
Wang D, Tai PWL, Gao G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat Rev Drug Discov. 2019;18:358–78.
Hu CJ, Lu YC, Tsai YH, Cheng HY, Takeda H, Huang CY, et al. Efficient in utero gene transfer to the Mammalian inner ears by the synthetic adeno-associated viral vector Anc80L65. Mol Ther Methods Clin Dev. 2020;18:493–500.
Tang H, Wang H, Wang S, Hu SW, Lv J, Xun M, et al. Hearing of Otof-deficient mice restored by trans-splicing of N- and C-terminal otoferlin. Human Genet. 2022; https://doi.org/10.1007/s00439-022-02504-2.
Wang Y, Menon N, Shen S, Feschenko M, Bergelson S. A qPCR method for AAV genome titer with ddPCR-level of accuracy and precision. Mol Ther Methods Clin Dev. 2020;19:341–6.
Hudry E, Martin C, Gandhi S, György B, Scheffer DI, Mu D, et al. Exosome-associated AAV vector as a robust and convenient neuroscience tool. Gene Ther. 2016;23:380–92.
Hanlon KS, Meltzer JC, Buzhdygan T, Cheng MJ, Sena-Esteves M, Bennett RE, et al. Selection of an efficient AAV vector for robust CNS transgene expression. Mol Ther Methods Clin Dev. 2019;15:320–32.
Askew C, Rochat C, Pan B, Asai Y, Ahmed H, Child E, et al. Tmc gene therapy restores auditory function in deaf mice. Sci Transl Med. 2015; https://doi.org/10.1126/scitranslmed.aab1996.
Burdett T, Nuseibeh S Changing trends in the development of AAV-based gene therapies: a meta-analysis of past and present therapies. Gene Ther. 2022; https://doi.org/10.1038/s41434-022-00363-0.
Zincarelli C, Soltys S, Rengo G, Rabinowitz JE. Analysis of AAV serotypes 1-9 mediated gene expression and tropism in mice after systemic injection. Mol Ther. 2008;16:1073–80.
Challis RC, Ravindra Kumar S, Chen X, Goertsen D, Coughlin GM, Hori AM, et al. Adeno-associated virus toolkit to target diverse brain cells. Annu Rev Neurosci. 2022;45:447–69.
Hudry E, Andres-Mateos E, Lerner EP, Volak A, Cohen O, Hyman BT, et al. Efficient gene transfer to the central nervous system by single-stranded Anc80L65. Mol Ther Methods Clin Dev. 2018;10:197–209.
Chakrabarty P, Rosario A, Cruz P, Siemienski Z, Ceballos-Diaz C, Crosby K, et al. Capsid serotype and timing of injection determines AAV transduction in the neonatal mice brain. PLoS One. 2013; https://doi.org/10.1371/journal.pone.0067680.
Yang B, Li S, Wang H, Guo Y, Gessler DJ, Cao C, et al. Global CNS transduction of adult mice by intravenously delivered rAAVrh.8 and rAAVrh.10 and nonhuman primates by rAAVrh.10. Mol Ther. 2014;22:1299–309.
Cayé-Thomasen P, Worsøe L, Brandt CT, Miyazaki H, Ostergaard C, Frimodt-Møller N, et al. Routes, dynamics, and correlates of cochlear inflammation in terminal and recovering experimental meningitis. Laryngoscope. 2009;119:1560–70.
Goertsen D, Flytzanis NC, Goeden N, Chuapoco MR, Cummins A, Chen Y, et al. AAV capsid variants with brain-wide transgene expression and decreased liver targeting after intravenous delivery in mouse and marmoset. Nat Neurosci. 2022;25:106–15.
Liu Y, Okada T, Nomoto T, Ke X, Kume A, Ozawa K, et al. Promoter effects of adeno-associated viral vector for transgene expression in the cochlea in vivo. Exp Mol Med. 2007;39:170–5.
Boëda B, Weil D, Petit C. A specific promoter of the sensory cells of the inner ear defined by transgenesis. Hum Mol Genet. 2001;10:1581–9. https://doi.org/10.1093/hmg/10.15.1581.
Shibata SB, Yoshimura H, Ranum PT, Goodwin AT, Smith RJH Intravenous rAAV2/9 injection for murine cochlear gene delivery. Sci Rep. 2017; https://doi.org/10.1038/s41598-017-09805-x.
Blanc F, Bemelmans AP, Affortit C, Joséphine C, Puel JL, Mondain M, et al. A single cisterna magna injection of AAV leads to binaural transduction in mice. Front Cell Dev Biol. 2022; https://doi.org/10.3389/fcell.2021.783504.
Talaei S, Schnee ME, Aaron KA, Ricci AJ. Dye tracking following posterior semicircular canal or round window membrane injections suggests a role for the Cochlea aqueduct in modulating distribution. Front Cell Neurosci. 2019; https://doi.org/10.3389/fncel.2019.00471.
Bankoti K, Generotti C, Hwa T, Wang L, O’Malley BW Jr, Li D. Advances and challenges in adeno-associated viral inner-ear gene therapy for sensorineural hearing loss. Mol Ther Methods Clin Dev. 2021;21:209–36. https://doi.org/10.1016/j.omtm.2021.03.005.
Baldrick P, McIntosh B, Prasad M. Adeno-associated virus (AAV)-based gene therapy products: what are toxicity studies in non-human primates showing us? Regul Toxicol Pharmacol. 2023;138:105332. https://doi.org/10.1016/j.yrtph.2022.105332.
György B, Meijer EJ, Ivanchenko MV, Tenneson K, Emond F, Hanlon KS, et al. Gene transfer with AAV9-PHP.B rescues hearing in a mouse model of Usher syndrome 3A and transduces hair cells in a non-human primate. Mol Ther Methods Clin Dev. 2018;13:1–13. https://doi.org/10.1016/j.omtm.2018.11.003.
Ivanchenko MV, Hanlon KS, Devine MK, Tenneson K, Emond F, Lafond JF, et al. Preclinical testing of AAV9-PHP.B for transgene expression in the non-human primate cochlea. Hear Res. 2020;394:107930. https://doi.org/10.1016/j.heares.2020.107930.
Ivanchenko MV, Hanlon KS, Hathaway DM, Klein AJ, Peters CW, Li Y, et al. AAV-S: A versatile capsid variant for transduction of mouse and primate inner ear. Mol Ther Methods Clin Dev. 2021;21:382–98. https://doi.org/10.1016/j.omtm.2021.03.019.
Acknowledgements
We thank Z.W. Gao for helping with the manuscript, L. Han for assistance with animal breeding.
Funding
This work was supported by the National Natural Science Foundation of China (82225014, 82171148, and 82192864), the National Key R&D Program of China (2020YFA0908201 and 2021YFA1101302), the Clinical Research Plan of SHDC (SHDC2020CR4083), the Science and Technology Commission of Shanghai (21S11905100), the Special Project for Clinical Research in Health Industry of Shanghai Municipal Health Commission (20224Z0003), the Shuguang Program of Shanghai Education Development Foundation and the Shanghai Municipal Education Commission (20SG08), and the Medical-Industrial Program of Fudan University, Natural Science Key Project of Scientific Research Innovation Program of Shanghai Education Commission (2023ZKZD12).
Author information
Authors and Affiliations
Contributions
SH, ZX designed and implemented experiments, generated figures, analyzed data, and wrote the manuscript. SH, ZX, and HW performed in RWM injections. SH contributed to hearing tests. ZX, and SW contributed to genomes quantification, SH and ZX contributed to VS-120 microscope and confocal microscopy experiments. HT and SH helped design experiments and write the manuscript. YS, and GG conceived and designed the project, supervised the research, and wrote the manuscript. All authors have reviewed and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Han, S., Xu, Z., Wang, S. et al. Distributional comparison of different AAV vectors after unilateral cochlear administration. Gene Ther 31, 154–164 (2024). https://doi.org/10.1038/s41434-023-00431-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41434-023-00431-z