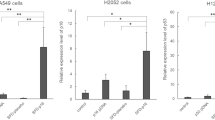
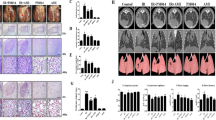
Abstract
Dry gene powder is a novel non-viral gene-delivery system, which is inhalable with high gene expression. Previously, we showed that the transfection of p16INK4a or TP53 by dry gene powder resulted in growth inhibitions of lung cancer and malignant pleural mesothelioma (MPM) in vitro and in vivo. Here, we report that dry gene powder containing p53- expression-plasmid DNA enhanced the therapeutic effects of cisplatin (CDDP) against MPM even in the presence of endogenous p53. Furthermore, our results indicated that the safe transfection with a higher plasmid DNA (pDNA) concentration suppressed MPM growth independently of chemotherapeutic agents. To develop a new therapeutic alternative for MPM patients without safety concerns over “vector doses”, our in vitro data provide basic understandings for dry gene powder.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Vogelzang NJ, Rusthoven JJ, Symanowski J, Denham C, Kaukel E, Ruffie P, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003;21:2636–44.
Mnohar S, Leung N. Cisplatin nephrotoxicity: a review of the literature. J Nephrol. 2018;31:15–25.
Rocha CRR, Silva MM, Quinet A, Cabral-Neto JB, Menck CFM. DNA repair pathways and cisplatin resistance: an intimate relationship. Clinics 2018;73:e478s.
Xu C, Hu Y, Chen B, Li D, Liang R, Shen M, et al. Metastasis-associated gene 1 (MTA1) enhances cisplatin resistance of malignant pleural mesothelioma by ATR-Chk1-mediated DNA repair. Ann Transl Med. 2021;9:670.
Giuliano M, Catalano A, Strizzi L, Vianale G, Capogrossi M, Procopio A. Adenovirus-mediated wild-type p53 over-expression reverts tumourigenicity of human mesothelioma cells. Int J Mol Med. 2000;5:591–6.
Li Q, Kawamura K, Yamanaka M, Okamoto S, Yang S, Yamauchi S, et al. Upregulated p53 expression activates apoptotic pathways in wild-type p53-bearing mesothelioma and enhances cytotoxicity of cisplatin and pemetrexed. Cancer Gene Ther. 2012;19:218–28.
Mohri K, Okuda T, Mori A, Danjo K, Okamoto H. Optimized pulmonary gene transfection in mice by spray–freeze dried powder inhalation. J Control Release. 2010;144:221–6.
Mizuno T, Mohri K, Nasu S, Danjo K, Okamoto H. Dual imaging of pulmonary delivery and gene expression of dry powder inhalant by fluorescence and bioluminescence. J Control Release. 2009;134:149–54.
Ito T, Okuda T, Takashima Y, Okamoto H. Naked pDNA inhalation powder composed of hyaluronic acid exhibits high gene expression in the lungs. Mol Pharm. 2019;16:489–97.
Asai A, Okuda T, Sonoda E, Yamauchi T, Kato S, Okamoto H. Drug permeation characterization of inhaled dry powder formulations in air-liquid interfaced cell layer using an improved, simple apparatus for dispersion. Pharm. Res. 2016;33:487–97.
Ito T, Okuda T, Takayama R, Okamoto H. Establishment of an evaluation method for gene silencing by serial pulmonary administration of siRNA and pDNA powders: naked siRNA inhalation powder suppresses luciferase gene expression in the lung. J Pharm Sci. 2019;108:2661–7.
Goodison S, Urquidi V, Tarin D. CD44 cell adhesion molecules. Mol Pathol. 1999;52:189–96.
Kim E, Yang J, Park J, Kim S, Kim NH, et al. Consecutive targetable smart nanoprobe for molecular recognition of cytoplasmic microRNA in metastatic breast cancer. ACS Nano. 2012;6:8525–35.
Zöller M. CD44: can a cancer-initiating cell profit from an abundantly expressed molecule? Nat Rev Cancer. 2011;11:254–67.
Attanoos RL, Dallimore NS, Gibbs AR. Primary epithelioid haemangioendothelioma of the peritoneum: an unusual mimic of diffuse malignant mesothelioma. Histopathology. 1997;30:375–7.
Stefano ID, Battaglia A, Zannoni GF, Prisco MG, Fattorossi A, Travaglia D, et al. Hyaluronic acid-paclitaxel: effects of intraperitoneal administration against CD44(+) human ovarian cancer xenografts. Cancer Chemother Pharmacol. 2011;68:107–16.
Bassi PF, Volpe A, D’Agostino D, Palermo G, Renier D, Franchini S, et al. Paclitaxel-hyaluronic acid for intravesical therapy of Bacillus Calmette-Guérin refractory carcinoma in situ of the bladder: results of a phase I study. J Urol. 2011;185:445–9.
Ito T, Fukuhara M, Okuda T, Okamoto H. Naked pDNA/hyaluronic acid powder shows excellent long-term storage stability and gene expression in murine lungs. Int J Pharm. 2020;574:118880.
Ichikawa M, Muramatsu N, Matsunaga W, Ishikawa T, Okuda T, Okamoto H, et al. Effects of inhalable gene transfection as a novel gene therapy for non-small cell lung cancer and malignant pleural mesothelioma. Sci Rep. 2022;12:1–8.
Guo G, Chmielecki J, Goparaju C, Heguy A, Dolgalev I, Carbone M, et al. Whole-exome sequencing reveals frequent genetic alterations in BAP1, NF2, CDKN2A, and CUL1 in malignant pleural mesothelioma. Pass Cancer Res. 2015;75:264–9.
Kadam P, Bhalerao S. Sample size calculation. Int J Ayurveda Res. 2010;1:55–7.
Davis MR, Manning LS, Whitaker D, Garlepp MJ, Robinson BW. Establishment of a murine model of malignant mesothelioma. Int J Cancer. 1992;52:881–6.
Wahlbuhl E, Liehr T, Rincic M, Azawi S. Cytogenomic characterization of three murine malignant mesothelioma tumor cell lines. Mol Cytogenet. 2020;13:43.
Taira N, Nihira K, Yamaguchi T, Miki Y, Yoshida K. DYRK2 is targeted to the nucleus and controls p53 via Ser46 phosphorylation in the apoptotic response to DNA damage. Mol Cell. 2007;25:725–38.
Olsson A, Manzl C, Strasser A, Villunger A. How important are posttranslational modifications in p53 for selectivity in target-gene transcription and tumour suppression? Cell Death Differ. 2007;14:1561–75.
Cecchinelli B, Porrello A, Lazzari C, Gradi A, Bossi G, D’Angelo M, et al. Ser58 of mouse p53 is the homologue of human Ser46 and is phosphorylated by HIPK2 in apoptosis. Cell Death Differ. 2006;13:1994–7.
Xia Y, Li X, Sun W. Applications of recombinant adenovirus-p53 gene therapy for cancers in the clinic in China. Curr Gene Ther. 2020;20:127–41.
Hosmani J, Mushtaq S, Abullais SS, Almubarak HM, Assiri K, Testarelli L, et al. Recombinant human adenovirus-p53 therapy for the treatment of oral leukoplakia and oral squamous cell carcinoma: a systematic review. Medicina. 2021;57:438.
Zhang W, Li L, Li D, Liu J, Li X, Li W, et al. The first approved gene therapy product for cancer Ad-p53(Gendicine):12 years in the clinic. Hum Gene Ther. 2018;29:160–79.
Keeler AM, Flotte TR. Recombinant adeno-associated virus gene therapy in light of Luxturna (and Zolgensma and Glybera): where are we, and how did we get here? Annu Rev Virol. 2019;6:601–21.
Wu Z, Asokan A, Samulski RJ. Adeno-associated virus serotypes: vector toolkit for human gene therapy. Mol Ther. 2006;14:316–27.
Teschendorf C, Emons B, Muzyczka N, Graeven U, Schmiegel W. Efficacy of recombinant adeno-associated viral vectors serotypes 1, 2, and 5 for the transduction of pancreatic and colon carcinoma cells. Anticancer Res. 2010;30:1931–5.
Melissa AK, Schaffer DV. Engineering adeno-associated viruses for clinical gene therapy. Nat Rev Genet. 2014;15:445–51.
Li Y, Guo W, Li X, Zhang J, Sun M, Tang Z, et al. Expert consensus on the clinical application of recombinant adenovirus human p53 for head and neck cancers. Int J Oral Sci. 2021;13:38.
Fujiwara T, Tanaka N, Kanazawa S, Ohtani S, Saijo Y, Nukiwa T, et al. Multicenter phase I study of repeated intratumoral delivery of adenoviral p53 in patients with advanced non-small-cell lung cancer. J Clin Oncol. 2006;24:1689–99.
Mendell JR, Al-Zaidy SA, Louise R, Rodino-Klapac LR, Goodspeed K, Gray SJ, et al. Current clinical applications of in vivo gene therapy with AAVs. Mol Ther. 2021;29:464–88.
High-dose AAV gene therapy deaths. Nat Biotechnol. 2020;38:910. https://doi.org/10.1038/s41587-020-0642-9.
Nishikawa H, Goto M, Fukunishi S, Asai A, Nishiguchi S, Higuchi K. Cancer cachexia: its mechanism and clinical significance.Int J Mol Sci. 2021;22:8491.
Cao K, Ding X, Sheng Y, Wang Y, Liu. Cisplatin binds to the MDM2 RING finger domain and inhibits the ubiquitination activity. Chem Commun. 2020;56:4599–602.
Nguyen TTT, Shingyoji M, Hanazono M, Zhong B, Morinaga T, Tada Y, et al. An MDM2 inhibitor achieves synergistic cytotoxic effects with adenoviruses lacking E1B55kDa gene on mesothelioma with the wild-type p53 through augmenting NFI expression. Cell Death Dis. 2021;12:663.
McConkey DJ. The integrated stress response and proteotoxicity in cancer therapy. Biochem Biophys Res Commun. 2017;482:450–3.
Brancolini C, Iuliano L. Proteotoxic stress and cell death in cancer cells. Cancers. 2020;12:2385.
Haraguchi T, Koujin T, Shindo T, Bilir S, Osakada H, Nishimura K, et al. Transfected plasmid DNA is incorporated into the nucleus via nuclear envelope reformation at telophase. Commun Biol. 2022;5:78.
Usami N, Fukui T, Kondo M, Taniguchi T, Yokoyama T, Mori S, et al. Establishment and characterization of four malignant pleural mesothelioma cell lines from Japanese patients. Cancer Sci. 2006;97:387–94.
Kastan MB, Onyekwere O, Sidransky D, Vogelstein B, Craig RW. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 1991;51:6304–11.
Leonardo AD, Linke SP, Clarkin K, Wahl GM. DNA damage triggers a prolonged p53-dependent G1 arrest and long-term induction of Cip1 in normal human fibroblasts. Genes Dev. 1994;8:2540–51.
Lindström MS, Bartek J, Maya-Mendoza A. p53 at the crossroad of DNA replication and ribosome biogenesis stress pathways. Cell Death Differ. 2022;29:972–82.
Taylor WR, Stark GR. Regulation of the G2/M transition by p53. Oncogene. 2001;20:1803–15.
Author information
Authors and Affiliations
Contributions
NM designed this study, collected the data, performed the analysis, and wrote the original draft. YT prepared the dry gene powder. YT, TK, MI, TO, and TH reviewed the manuscript. HO participated in the study design, revised the manuscript, and gave final approval of the version to be submitted. The work reported in the paper has been performed by the authors, unless clearly specified in the text. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
As this study does not involve animal studies, human subjects, human material, or human data, ethical approval is not applicable.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Muramatsu, N., Ichikawa, M., Katagiri, T. et al. p53 dry gene powder enhances anti-cancer effects of chemotherapy against malignant pleural mesothelioma. Gene Ther 31, 119–127 (2024). https://doi.org/10.1038/s41434-023-00424-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41434-023-00424-y