Abstract
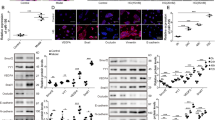
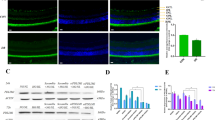
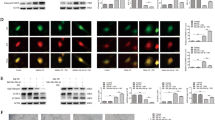
Diabetic retinopathy (DR) is a common microvascular complication. Many studies have focused on the role of microRNAs (miRNAs) in DR but not specifically on miR-133b-3p. Thus, this study is to unmask the mechanisms of miR-133b-3p in DR. KK/Upj-Ay mice (a spontaneous diabetic nephropathy model of DM, referred to as DR mice) were used in the study, and retinal tissues were collected. Bone marrow mesenchymal stem cells (BMSCs) were isolated and identified. High glucose (HG)-treated mouse retinal microvascular endothelial cells (mRMECs) were transfected or co-cultured with BMSCs-derived exosomes. Then, cell proliferation, migration, apoptosis, angiogenesis, and oxidative stress were observed. MiR-133b-3p and FBN1 expression in tissues and cells was detected. MiR-133b-3p expression was reduced, and FBN1 expression was increased in retinal tissues of DR mice and HG-treated mRMECs. Up-regulating miR-133b-3p or down-regulating FBN1 or BMSCs-derived exosomes impaired oxidative stress, angiogenesis, proliferation, migration, and promoted apoptosis of HG-treated mRMECs. This study has elucidated that exosomal miR-133b-3p from BMSCs suppresses angiogenesis and oxidative stress in DR via FBN1 repression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Cunha-Vaz J, Ribeiro L, Lobo C. Phenotypes and biomarkers of diabetic retinopathy. Prog Retin Eye Res. 2014;41:90–111.
Sabanayagam C, Banu R, Chee ML, Lee R, Wang YX, Tan G, et al. Incidence and progression of diabetic retinopathy: a systematic review. Lancet Diabetes Endocrinol. 2019;7:140–9.
May M, Framke T, Junker B, Framme C, Pielen A, Schindler C. How and why SGLT2 inhibitors should be explored as potential treatment option in diabetic retinopathy: clinical concept and methodology. Ther Adv Endocrinol Metab. 2019;10:2042018819891886.
Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35:556–64.
Anwar MS, Shakaib B, Akhtar W, Yusufzai E, Zehra M, Munawar H, et al. Knowledge and practices of primary care physicians on the current referral system of diabetic retinopathy in Islamabad and Rawal-Pindi, Pakistan. Int J Ophthalmol. 2019;12:1885–92.
Shantikumar S, Caporali A, Emanueli C. Role of microRNAs in diabetes and its cardiovascular complications. Cardiovasc Res. 2012;93:583–93.
Esteves JV, Enguita FJ, Machado UF. MicroRNAs-mediated regulation of skeletal muscle GLUT4 expression and translocation in insulin resistance. J Diabetes Res. 2017;2017:7267910.
Yao J, Wang J, Yao Y, Wang K, Zhou Q, Tang Y. miR133b regulates proliferation and apoptosis in highglucoseinduced human retinal endothelial cells by targeting ras homolog family member A. Int J Mol Med. 2018;42:839–50.
Huang C, Fisher KP, Hammer SS, Navitskaya S, Blanchard GJ, Busik JV. Plasma exosomes contribute to microvascular damage in diabetic retinopathy by activating the classical complement pathway. Diabetes. 2018;67:1639–49.
Zhang Y, Chopp M, Zhang ZG, Katakowski M, Xin H, Qu C, et al. Systemic administration of cell-free exosomes generated by human bone marrow derived mesenchymal stem cells cultured under 2D and 3D conditions improves functional recovery in rats after traumatic brain injury. Neurochem Int. 2017;111:69–81.
Mariko B, Pezet M, Escoubet B, Bouillot S, Andrieu JP, Starcher B, et al. Fibrillin-1 genetic deficiency leads to pathological ageing of arteries in mice. J Pathol. 2011;224:33–44.
Hubmacher D, Reinhardt DP, Plesec T, Schenke-Layland K, Apte SS. Human eye development is characterized by coordinated expression of fibrillin isoforms. Invest Ophthalmol Vis Sci. 2014;55:7934–44.
Lang FM, Hossain A, Gumin J, Momin EN, Shimizu Y, Ledbetter D, et al. Mesenchymal stem cells as natural biofactories for exosomes carrying miR-124a in the treatment of gliomas. Neuro Oncol. 2018;20:380–90.
Yang D, Zhao D, Chen X. MiR-133b inhibits proliferation and invasion of gastric cancer cells by up-regulating FBN1 expression. Cancer Biomark. 2017;19:425–36.
Costa V, Ciccodicola A. Is PPARG the key gene in diabetic retinopathy? Br J Pharmacol. 2012;165:1–3.
Gong Q, Xie J, Liu Y, Li Y, Su G. Differentially expressed MicroRNAs in the development of early diabetic retinopathy. J Diabetes Res. 2017;2017:4727942.
Yildirim SS, Akman D, Catalucci D, Turan B. Relationship between downregulation of miRNAs and increase of oxidative stress in the development of diabetic cardiac dysfunction: junctin as a target protein of miR-1. Cell Biochem Biophys. 2013;67:1397–408.
Zhao Y, Huang J, Zhang L, Qu Y, Li J, Yu B, et al. MiR-133b is frequently decreased in gastric cancer and its overexpression reduces the metastatic potential of gastric cancer cells. BMC Cancer. 2014;14:34.
Wei Y, He R, Wu Y, Gan B, Wu P, Qiu X, et al. Comprehensive investigation of aberrant microRNA profiling in bladder cancer tissues. Tumour Biol. 2016;37:12555–69.
Li C, Liu Z, Yang K, Chen X, Zeng Y, Liu J, et al. miR-133b inhibits glioma cell proliferation and invasion by targeting Sirt1. Oncotarget. 2016;7:36247–54.
Liu TT, Hao Q, Zhang Y, Li ZH, Cui ZH, Yang W. Effects of microRNA-133b on retinal vascular endothelial cell proliferation and apoptosis through angiotensinogen-mediated angiotensin II- extracellular signal-regulated kinase 1/2 signalling pathway in rats with diabetic retinopathy. Acta Ophthalmol. 2018;96:e626–e635.
Marek I, Volkert G, Hilgers KF, Bieritz B, Rascher W, Reinhardt DP, et al. Fibrillin-1 and alpha8 integrin are co-expressed in the glomerulus and interact to convey adhesion of mesangial cells. Cell Adh Migr. 2014;8:389–95.
Xu C, Hu Y, Hou L, Ju J, Li X, Du N, et al. beta-Blocker carvedilol protects cardiomyocytes against oxidative stress-induced apoptosis by up-regulating miR-133 expression. J Mol Cell Cardiol. 2014;75:111–21.
Gu Y, Liang Z, Wang H, Jin J, Zhang S, Xue S, et al. Tanshinone IIA protects H9c2 cells from oxidative stress-induced cell death via microRNA-133 upregulation and Akt activation. Exp Ther Med. 2016;12:1147–52.
Xu H, Zaidi M, Struve J, Jones DW, Krolikowski JG, Nandedkar S, et al. Abnormal fibrillin-1 expression and chronic oxidative stress mediate endothelial mesenchymal transition in a murine model of systemic sclerosis. Am J Physiol Cell Physiol. 2011;300:C550–6.
Yang J, Liu XX, Fan H, Tang Q, Shou ZX, Zuo DM, et al. Extracellular vesicles derived from bone marrow mesenchymal stem cells protect against experimental colitis via attenuating colon inflammation, oxidative stress and apoptosis. PLoS One. 2015;10:e0140551.
Pan JY, Sun CC, Bi ZY, Chen ZL, Li SJ, Li QQ, et al. miR-206/133b cluster: a weapon against lung cancer? Mol Ther Nucleic Acids. 2017;8:442–9.
Huang H, Xu Y, Guo Z, Chen X, Ji S, Xu Z. MicroRNA-133b inhibits cell proliferation and promotes apoptosis by targeting cullin 4B in esophageal squamous cell carcinoma. Exp Ther Med. 2018;15:3743–50.
Chang L, Lei X, Qin YU, Zhang X, Jin H, Wang C, et al. MicroRNA-133b inhibits cell migration and invasion by targeting matrix metalloproteinase 14 in glioblastoma. Oncol Lett. 2015;10:2781–6.
Li B, Ding CM, Li YX, Peng JC, Geng N, Qin WW. Over-regulation of microRNA-133b inhibits cell proliferation of cisplatin-induced non-small cell lung cancer cells through PI3K/Akt and JAK2/STAT3 signaling pathway by targeting EGFR. Oncol Rep. 2018;39:1227–34.
Li H, Xiang Z, Liu Y, Xu B, Tang J. MicroRNA-133b inhibits proliferation, cellular migration, and invasion via targeting lasp1 in hepatocarcinoma cells. Oncol Res. 2017;25:1269–82.
Ma X, Wei J, Zhang L, Deng D, Liu L, Mei X, et al. miR-486-5p inhibits cell growth of papillary thyroid carcinoma by targeting fibrillin-1. Biomed Pharmacother. 2016;80:220–6.
Acknowledgements
We would like to acknowledge the reviewers for their helpful comments on this paper.
Funding
This work was supported by the Natural Science Foundation of Guangxi (Grant/Award number:2020GXNSFAA259054) and First Batch of High-level Talent Scientific Research Projects of the Affiliated Hospital of Youjiang Medical University for Nationalities in 2019 (Grant/Award number:R20196340 & Y20196304)).
Author information
Authors and Affiliations
Contributions
WM contributed to study design; GL contributed to manuscript editing; ZQ, YL, and JY contributed to experimental studies; ZS and RW contributed to data analysis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Liang, G., Qin, Z., Luo, Y. et al. Exosomal microRNA-133b-3p from bone marrow mesenchymal stem cells inhibits angiogenesis and oxidative stress via FBN1 repression in diabetic retinopathy. Gene Ther 29, 710–719 (2022). https://doi.org/10.1038/s41434-021-00310-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41434-021-00310-5