Abstract
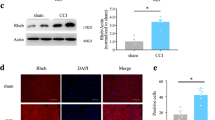
Neuropathic pain is a somatosensory nervous system dysfunction that remains a threatening health problem globally. Recent studies have highlighted the involvement of C-C motif chemokine receptor 1 (CCR1) in neuropathic pain. Herein, the current study set out to explore the modulatory role of CCR1 in spinal nerve ligation (SNL)-induced neuropathic pain and its underlying molecular mechanism. First, it was found that CCR1 was highly expressed in spinal cord tissues and microglial cells of SNL rats. On the other hand, CCR1 knockdown attenuated nerve pain in SNL rats and repressed microglial cell activation in SNL rats and also in the LPS-induced microglial cell model of nerve injury, as evidenced by elevated microglial cell markers OX-42 and IL-1β, IL-6 and TNF-α. Mechanistically, CCR1 enhanced small ubiquitin-like modifier 1 (SUMO1) modification of DiGeorge syndrome critical region gene 8 (DGCR8) in LPS-treated microglial cells by phosphorylating ERK. Moreover, CCR1 silencing brought about elevations in mechanical withdrawal threshold and thermal withdrawal latency. To conclude, our findings indicated that CCR1 enhanced the modification of DGCR8 by SUMO1 through phosphorylation of ERK, thereby promoting the activation and inflammatory response of spinal cord microglial cells and increasing the sensitivity of SNL rats to pain. Thus, this study offers a promising therapeutic target for the management of neuropathic pain.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Hamad MK, He K, Abdulrazeq HF, Mustafa AM, Luceri R, Kamal N, et al. Potential uses of isolated toxin peptides in neuropathic pain relief: a literature review. World Neurosurg. 2018;113:333–47 e5.
Machado-Duque ME, Gaviria-Mendoza A, Machado-Alba JE, Castano N. Evaluation of treatment patterns and direct costs associated with the management of neuropathic pain. Pain Res Manag. 2020;2020:9353940.
Cavalli E, Mammana S, Nicoletti F, Bramanti P, Mazzon E. The neuropathic pain: an overview of the current treatment and future therapeutic approaches. Int J Immunopathol Pharmacol. 2019;33:2058738419838383.
Bouhassira D, Attal N. Emerging therapies for neuropathic pain: new molecules or new indications for old treatments? Pain. 2018;159:576–82.
Mosser CA, Baptista S, Arnoux I, Audinat E. Microglia in CNS development: shaping the brain for the future. Prog Neurobiol. 2017;149-150:1–20.
Borgonetti V, Galeotti N. Combined inhibition of histone deacetylases and BET family proteins as epigenetic therapy for nerve injury-induced neuropathic pain. Pharmacol Res. 2021;165:105431.
Yi MH, Liu YU, Liu K, Chen T, Bosco DB, Zheng J, et al. Chemogenetic manipulation of microglia inhibits neuroinflammation and neuropathic pain in mice. Brain Behav Immun. 2021;92:78–89.
Liou JT, Lee CM, Day YJ. The immune aspect in neuropathic pain: role of chemokines. Acta Anaesthesiol Taiwan. 2013;51:127–32.
Balasubramanian PK, Balupuri A, Kothandan G, Cho SJ. In silico study of 1-(4-Phenylpiperazin-1-yl)-2-(1H-pyrazol-1-yl) ethanones derivatives as CCR1 antagonist: homology modeling, docking and 3D-QSAR approach. Bioorg Med Chem Lett. 2014;24:928–33.
Pawlik K, Piotrowska A, Kwiatkowski K, Ciapala K, Popiolek-Barczyk K, Makuch W, et al. The blockade of CC chemokine receptor type 1 influences the level of nociceptive factors and enhances opioid analgesic potency in a rat model of neuropathic pain. Immunology. 2020;159:413–28.
Yan J, Zuo G, Sherchan P, Huang L, Ocak U, Xu W, et al. CCR1 activation promotes neuroinflammation through CCR1/TPR1/ERK1/2 signaling pathway after intracerebral hemorrhage in mice. Neurotherapeutics. 2020;17:1170–83.
Lu N, Malemud CJ. Extracellular Signal-Regulated Kinase: A Regulator of Cell Growth, Inflammation, Chondrocyte and Bone Cell Receptor-Mediated Gene Expression. Int J Mol Sci. 2019;20:3792. https://doi.org/10.3390/ijms20153792.
Chen Y, Yin D, Fan B, Zhu X, Chen Q, Li Y, et al. Chemokine CXCL10/CXCR3 signaling contributes to neuropathic pain in spinal cord and dorsal root ganglia after chronic constriction injury in rats. Neurosci Lett. 2019;694:20–8.
Herbert KM, Pimienta G, DeGregorio SJ, Alexandrov A, Steitz JA. Phosphorylation of DGCR8 increases its intracellular stability and induces a progrowth miRNA profile. Cell Rep. 2013;5:1070–81.
Daniel JA, Cooper BH, Palvimo JJ, Zhang FP, Brose N, Tirard M. Analysis of SUMO1-conjugation at synapses. Elife. 2017;6:e26338. https://doi.org/10.7554/eLife.26338.
Huang YG, Zhang Q, Wu H, Zhang CQ. A comparison of surgical invasions for spinal nerve ligation with or without paraspinal muscle removal in a rat neuropathic pain model. Biomed Res Int. 2016;2016:6741295.
Vincenzetti S, Pucciarelli S, Huang Y, Ricciutelli M, Lambertucci C, Volpini R, et al. Biomarkers mapping of neuropathic pain in a nerve chronic constriction injury mice model. Biochimie. 2019;158:172–9.
Zhang ZJ, Jiang BC, Gao YJ. Chemokines in neuron-glial cell interaction and pathogenesis of neuropathic pain. Cell Mol Life Sci. 2017;74:3275–91.
Kwiatkowski K, Mika J. The importance of chemokines in neuropathic pain development and opioid analgesic potency. Pharmacol Rep. 2018;70:821–30.
Tozaki-Saitoh H, Tsuda M. Microglia-neuron interactions in the models of neuropathic pain. Biochem Pharmacol. 2019;169:113614.
Inkinen S, Hakkarainen M, Albertsson AC, Sodergard A. From lactic acid to poly(lactic acid) (PLA): characterization and analysis of PLA and its precursors. Biomacromolecules. 2011;12:523–32.
Furukawa S, Hayashi S, Usuda K, Abe M, Ogawa I. The impairment of metrial gland development in tamoxifen exposed rats. Exp Toxicol Pathol. 2012;64:121–6.
Ge Y, Huang M, Yao YM. Autophagy and proinflammatory cytokines: interactions and clinical implications. Cytokine Growth Factor Rev. 2018;43:38–46.
Jin D, Yang JP, Hu JH, Wang LN, Zuo JL. MCP-1 stimulates spinal microglia via PI3K/Akt pathway in bone cancer pain. Brain Res. 2015;1599:158–67.
Guo D, Hu X, Zhang H, Lu C, Cui G, Luo X. Orientin and neuropathic pain in rats with spinal nerve ligation. Int Immunopharmacol. 2018;58:72–9.
Maruta T, Nemoto T, Hidaka K, Koshida T, Shirasaka T, Yanagita T, et al. Upregulation of ERK phosphorylation in rat dorsal root ganglion neurons contributes to oxaliplatin-induced chronic neuropathic pain. PLoS One. 2019;14:e0225586.
Xu X, Fu S, Shi X, Liu R. Microglial BDNF, PI3K, and p-ERK in the spinal cord are suppressed by pulsed radiofrequency on dorsal root ganglion to ease SNI-induced neuropathic pain in rats. Pain Res Manag. 2019;2019:5948686.
Vallet S, Pozzi S, Patel K, Vaghela N, Fulciniti MT, Veiby P, et al. A novel role for CCL3 (MIP-1alpha) in myeloma-induced bone disease via osteocalcin downregulation and inhibition of osteoblast function. Leukemia. 2011;25:1174–81.
Yuan H, Deng R, Zhao X, Chen R, Hou G, Zhang H, et al. SUMO1 modification of KHSRP regulates tumorigenesis by preventing the TL-G-Rich miRNA biogenesis. Mol Cancer. 2017;16:157.
Zhu C, Chen C, Huang J, Zhang H, Zhao X, Deng R, et al. SUMOylation at K707 of DGCR8 controls direct function of primary microRNA. Nucleic Acids Res. 2015;43:7945–60.
Funding
The research was supported by Project supported by the National Natural Science Foundation of China (Grant No. 82002387).
Author information
Authors and Affiliations
Contributions
CXS, JJ, YGX and ZL designed the study. JHM and TL were involved in data collection and statistical analysis. HYX and ZL prepared the figures. CXS, JJ, HYX and YGX drafted the paper. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Shi, C., Jin, J., Xu, H. et al. CCR1 enhances SUMOylation of DGCR8 by up-regulating ERK phosphorylation to promote spinal nerve ligation-induced neuropathic pain. Gene Ther 29, 379–389 (2022). https://doi.org/10.1038/s41434-021-00285-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41434-021-00285-3