Abstract
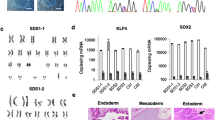
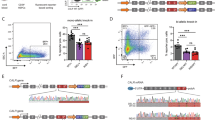
Induced pluripotent stem cells (iPSCs) from patients with genetic disorders are a valuable source for in vitro disease models, which enable drug testing and validation of gene and cell therapies. We generated iPSCs from a severe congenital neutropenia (SCN) patient, who presented with a nonsense mutation in the glucose-6-phosphatase catalytic subunit 3 (G6PC3) gene causing profound defects in granulopoiesis, associated with increased susceptibility of neutrophils to apoptosis. Generated SCN iPSC clones exhibited the capacity to differentiate into hematopoietic cells of the myeloid lineage and we identified two cytokine conditions, i.e., using granulocyte-colony stimulating factor or granulocyte-macrophage colony stimulating factor in combination with interleukin-3, to model the SCN phenotype in vitro. Reduced numbers of granulocytes were produced by SCN iPSCs compared with control iPSCs in both settings, which reflected the phenotype in patients. Interestingly, our model showed increased monocyte/macrophage production from the SCN iPSCs. Most importantly, lentiviral genetic correction of SCN iPSCs with a codon-optimized G6PC3 transgene restored granulopoiesis and reduced apoptosis of in vitro differentiated myeloid cells. Moreover, addition of vitamin B3 clearly induced granulocytic differentiation of SCN iPSCs and increased the number of neutrophils to levels comparable with those obtained from healthy control iPSCs. In summary, we established an iPSC-derived in vitro disease model, which will serve as a tool to test the potency of alternative treatment options for SCN patients, such as small molecules and gene therapeutic vectors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
Klein C. Genetic defects in severe congenital neutropenia: emerging insights into life and death of human neutrophil granulocytes. Annu Rev Immunol. 2011;29:399–413.
Skokowa J, Dale DC, Touw IP, Zeidler C, Welte K. Severe congenital neutropenias. Nat Rev Dis Primer. 2017;3:17032.
Freedman MH, Bonilla MA, Fier C, Bolyard AA, Scarlata D, Boxer LA, et al. Myelodysplasia syndrome and acute myeloid leukemia in patients with congenital neutropenia receiving G-CSF therapy. Blood. 2000;96:429–36.
Rosenberg PS, Zeidler C, Bolyard AA, Alter BP, Bonilla MA, Boxer LA, et al. Stable long-term risk of leukaemia in patients with severe congenital neutropenia maintained on G-CSF therapy. Br J Haematol. 2010;150:196–9.
De Ravin SS, Wu X, Moir S, Kardava L, Anaya-O’Brien S, Kwatemaa N, et al. Lentiviral hematopoietic stem cell gene therapy for X-linked severe combined immunodeficiency. Sci Transl Med. 2016;8:335ra57.
Hacein-Bey-Abina S, Pai S-Y, Gaspar HB, Armant M, Berry CC, Blanche S, et al. A modified γ-retrovirus vector for X-linked severe combined immunodeficiency. N Engl J Med. 2014;371:1407–17.
Aiuti A, Biasco L, Scaramuzza S, Ferrua F, Cicalese MP, Baricordi C, et al. Lentiviral hematopoietic stem cell gene therapy in patients with Wiskot–Aldrich syndrome. Science. 2013;341:1233151.
Braun CJ, Boztug K, Paruzynski A, Witzel M, Schwarzer A, Rothe M, et al. Gene therapy for Wiskott-Aldrich syndrome—long-term efficacy and genotoxicity. Sci Transl Med. 2014;6:227ra33.
Nanua S, Murakami M, Xia J, Grenda DS, Woloszynek J, Strand M, et al. Activation of the unfolded protein response is associated with impaired granulopoiesis in transgenic mice expressing mutant Elane. Blood. 2011;117:3539–47.
Cheung YY, Kim SY, Yiu WH, Pan C-J, Jun H-S, Ruef RA, et al. Impaired neutrophil activity and increased susceptibility to bacterial infection in mice lacking glucose-6-phosphatase–β. J Clin Investig. 2007;117:784–93.
Nayak RC, Trump LR, Aronow BJ, Myers K, Mehta P, Kalfa T, et al. Pathogenesis of ELANE-mutant severe neutropenia revealed by induced pluripotent stem cells. J Clin Investig. 2015;125:3103–16.
Hiramoto T, Ebihara Y, Mizoguchi Y, Nakamura K, Yamaguchi K, Ueno K, et al. Wnt3a stimulates maturation of impaired neutrophils developed from severe congenital neutropenia patient-derived pluripotent stem cells. Proc Natl Acad Sci USA. 2013;110:3023–8.
Dannenmann B, Zahabi A, Mir P, Oswald B, Bernhard R, Klimiankou M, et al. Human iPSC-based model of severe congenital neutropenia reveals elevated UPR and DNA damage in CD34+ cells preceding leukemic transformation. Exp Hematol. 2019;71:51–60.
Morishima T, Watanabe K-i, Niwa A, Hirai H, Saida S, Tanaka T, et al. Genetic correction of HAX1 in induced pluripotent stem cells from a patient with severe congenital neutropenia improves defective granulopoiesis. Haematologica. 2014;99:19–27.
Pittermann E, Lachmann N, Maclean G, Emmrich S, Ackermann M, Ohring G, et al. Gene correction of HAX1 reversed Kostmann disease phenotype in patient-specific induced pluripotent stem cells. Blood Adv. 2017;1:903–14.
Satoh D, Maeda T, Ito T, Nakajima Y, Ohte M, Ukai A, et al. Establishment and directed differentiation of induced pluripotent stem cells from glycogen storage disease type Ib patient. Genes Cells. 2013;18:1053–69.
Banka S, Newman WG. A clinical and molecular review of ubiquitous glucose-6-phosphatase deficiency caused by G6PC3 mutations. Orphanet J Rare Dis. 2013;8:1.
Boztug K, Appaswamy G, Ashikov A, Schäffer AA, Salzer U, Diestelhorst J, et al. A syndrome with congenital neutropenia and mutations in G6PC3. N Engl J Med. 2009;360:32–43.
Hoffmann D, Schott JW, Geis FK, Lange L, Müller F-J, Lenz D, et al. Detailed comparison of retroviral vectors and promoter configurations for stable and high transgene expression in human induced pluripotent stem cells. Gene Ther. 2017;24:298–307.
Dull T, Zufferey R, Kelly M, Mandel RJ, Nguyen M, Trono D, et al. A third-generation lentivirus vector with a conditional packaging system. J Virol. 1998;72:8463–71.
Ai HW, Shaner NC, Cheng Z, Tsien RY, Campbell RE. Exploration of new chromophore structures leads to the identification of improved blue fluorescent proteins. Biochemistry. 2007;46:5904–10.
Lachmann N, Ackermann M, Frenzel E, Liebhaber S, Brennig S, Happle C, et al. Large-scale hematopoietic differentiation of human induced pluripotent stem cells provides granulocytes or macrophages for cell replacement therapies. Stem Cell Rep. 2015;4:282–96.
Warlich E, Kuehle J, Cantz T, Brugman MH, Maetzig T, Galla M, et al. Lentiviral vector design and imaging approaches to visualize the early stages of cellular reprogramming. Mol Ther. 2011;19:782–9.
Voelkel C, Galla M, Maetzig T, Warlich E, Kuehle J, Zychlinski D, et al. Protein transduction from retroviral Gag precursors. Proc Natl Acad Sci USA. 2010;107:7805–10.
Rothe M, Rittelmeyer I, Iken M, Rüdrich U, Schambach A, Glage S, et al. Epidermal growth factor improves lentivirus vector gene transfer into primary mouse hepatocytes. Gene Ther. 2012;19:425–34.
Naldini L. Gene therapy returns to centre stage. Nature. 2015;526:351–60.
Müller-Kuller U, Ackermann M, Kolodziej S, Brendel C, Fritsch J, Lachmann N, et al. A minimal ubiquitous chromatin opening element (UCOE) effectively prevents silencing of juxtaposed heterologous promoters by epigenetic remodeling in multipotent and pluripotent stem cells. Nucleic Acids Res. 2015;43:1577–92.
Donadieu J, Fenneteau O, Beaupain B, Mahlaoui N, Chantelot C. Congenital neutropenia: diagnosis, molecular bases and patient management. Orphanet J Rare Dis. 2011;6:26.
Koch C, Samareh B, Morishima T, Mir P, Kanz L, Zeidler C, et al. GM-CSF treatment is not effective in congenital neutropenia patients due to its inability to activate NAMPT signaling. Ann Hematol. 2017;96:345–53.
Skokowa J, Lan D, Thakur BK, Wang F, Gupta K, Cario G, et al. NAMPT is essential for the G-CSF-induced myeloid differentiation via a NAD+-sirtuin-1-dependent pathway. Nat Med. 2009;15:151–8.
Notarangelo LD, Savoldi G, Cavagnini S, Bennato V, Vasile S, Pilotta A, et al. Severe congenital neutropenia due to G6PC3 deficiency: early and delayed phenotype in two patients with two novel mutations. Ital J Pediatr. 2014;40:80.
Jun HS, Cheung YY, Lee YM, Mansfield BC, Chou JY. Glucose-6-phosphatase-β, implicated in a congenital neutropenia syndrome, is essential for macrophage energy homeostasis and functionality. Blood. 2012;119:4047–55.
Mistry A, Scambler T, Parry D, Wood M, Barcenas-Morales G, Carter C, et al. Glucose-6-phosphatase catalytic subunit 3 (G6PC3) deficiency associated with autoinflammatory complications. Front Immunol. 2017;8:1485.
Boztug K, Rosenberg PS, Dorda M, Banka S, Moulton T, Curtin J, et al. Extended spectrum of human glucose-6-phosphatase catalytic subunit 3 deficiency: novel genotypes and phenotypic variability in severe congenital neutropenia. J Pediatr. 2012;160:679.
Ackermann M, Kempf H, Hetzel M, Hesse C, Hashtchin AR, Brinkert K, et al. Bioreactor-based mass production of human iPSC-derived macrophages enables immunotherapies against bacterial airway infections. Nat Commun. 2018;9:5088.
Cugno C, Deola S, Filippini P, Stroncek DF, Rutella S. Granulocyte transfusions in children and adults with hematological malignancies: benefits and controversies. J Transl Med. 2015;13:362.
Trump LR, Nayak RC, Singh AK, Emberesh S, Wellendorf AM, Lutzko CM, et al. Neutrophils derived from genetically modified human induced pluripotent stem cells circulate and phagocytose bacteria in vivo. Stem Cells Transl Med. 2019;8:557–67.
Acknowledgements
This work was supported by grants from the Bundesministerium für Bildung und Forschung (PID-NET FK2016M1517F; MyPred), the Deutsche Forschungsgemeinschaft (Cluster of Excellence REBIRTH (EXC 62/2) and SFB738), and the Deutsche Akademische Austauschdienst (DAAD). We thank Gerald Draeger and Thomas Scheper for providing Rock inhibitor Y-27632 and bFGF (Leibniz University Hannover, Hannover, Germany) and Malte Sgodda and Tobias Cantz for H9 ESC RNA (Hannover Medical School, Hannover, Germany), and Robert E. Campbell (University of Alberta, Alberta, Canada) for providing EBFP2.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Hoffmann, D., Kuehle, J., Lenz, D. et al. Lentiviral gene therapy and vitamin B3 treatment enable granulocytic differentiation of G6PC3-deficient induced pluripotent stem cells. Gene Ther 27, 297–306 (2020). https://doi.org/10.1038/s41434-020-0127-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41434-020-0127-y
This article is cited by
-
SLGT2 Inhibitor Rescues Myelopoiesis in G6PC3 Deficiency
Journal of Clinical Immunology (2022)
-
Differentiation of human induced pluripotent stem cells into erythroid cells
Stem Cell Research & Therapy (2020)