Abstract
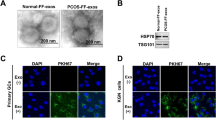
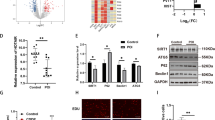
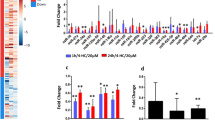
Increasing microRNAs are shown to be participate in polycystic ovarian syndrome (PCOS) pathogenesis. Nevertheless, the biological effects of miR-144-3p and its detailed mechanisms in PCOS are to be investigated. The purpose of our work was to study the function of miR-144-3p in PCOS. Currently, Expression of miR-144-3p was greatly reduced in PCOS patients and PCOS rat models. In addition, HSP-70 expression was greatly elevated PCOS. Cell proliferation assays and flow cytometry assay were carried out following the overexpression of miR-144-3p in ovarian granulosa cells from PCOS rat models. We observed that miR-144-3p overexpression induced the proliferation and repressed cell apoptosis while loss of miR-144-3p demonstrated an opposite process. Then, PCOS rat models were classified to four groups: LV-NC group, LV-miR-144-3p group, Anti-control group, and Anti-miR-144-3p group. In response to loss of miR-144-3p, we found E2, T, and LH serum levels were elevated and FSH serum level was inhibited. Upregulation of miR-144-3p exhibited an opposite process. Moreover, HSP-70 was a direct target of miR-144-3p. Furthermore, increased expression of HSP-70 rescued the effects of miR-144-3p on ovarian granulosa cell growth and apoptosis. In addition, knockdown of HSP-70 alleviated endocrine disorders and abnormal ovarian weight in vivo. To sum up, miR-144-3p might function as a novel target for PCOS treatment via targeting HSP-70.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The data are available upon reasonable request.
References
Madnani N, Khan K, Chauhan P, Parmar G. Polycystic ovarian syndrome. Indian J Dermatol Venereol Leprol. 2013;79:310–21.
Witchel SF, Oberfield SE, Pena AS. Polycystic ovary syndrome: pathophysiology, presentation, and treatment with emphasis on adolescent girls. J Endocrine Soc. 2019;3:1545–73.
Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, et al. Positions statement: criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: an Androgen Excess Society guideline. J Clin Endocrinol. Metab. 2006;91:4237–45.
Trikudanathan S. Polycystic ovarian syndrome. Med Clinics North Am. 2015;99:221–35.
Das M, Djahanbakhch O, Hacihanefioglu B, Saridogan E, Ikram M, Ghali L, et al. Granulosa cell survival and proliferation are altered in polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93:881–7.
Erickson GF, Magoffin DA, Garzo VG, Cheung AP, Chang RJ. Granulosa cells of polycystic ovaries: are they normal or abnormal? Hum Reprod. 1992;7:293–9.
Ying SY, Chang DC, Miller JD, Lin SL. The microRNA: overview of the RNA gene that modulates gene functions. Methods Mol Biol. 2006;342:1–18.
Bhaskaran M, Mohan M. MicroRNAs: history, biogenesis, and their evolving role in animal development and disease. Veterinary Pathol. 2014;51:759–74.
Dehwah MA, Xu A, Huang Q. MicroRNAs and type 2 diabetes/obesity. J Gen Gnom. 2012;39:11–8.
Sang Q, Yao Z, Wang H, Feng R, Wang H, Zhao X, et al. Identification of microRNAs in human follicular fluid: characterization of microRNAs that govern steroidogenesis in vitro and are associated with polycystic ovary syndrome in vivo. J Clin Endocrinol Metab. 2013;98:3068–79.
Sorensen AE, Wissing ML, Salo S, Englund AL, Dalgaard LT. MicroRNAs related to polycystic ovary syndrome (PCOS). Genes. 2014;5:684–708.
He T, Sun Y, Zhang Y, Zhao S, Zheng Y, Hao G, et al. MicroRNA-200b and microRNA-200c are up-regulated in PCOS granulosa cell and inhibit KGN cell proliferation via targeting PTEN. Rep Biol Endocrinol. 2019;17:68.
Fu X, He Y, Wang X, Peng D, Chen X, Li X, et al. MicroRNA-16 promotes ovarian granulosa cell proliferation and suppresses apoptosis through targeting PDCD4 in polycystic ovarian syndrome. Cell Physiol Biochem. 2018;48:670–82.
Han XM, Tian PY, Zhang JL. MicroRNA-486-5p inhibits ovarian granulosa cell proliferation and participates in the development of PCOS via targeting MST4. Eur Rev Med Pharmacol Sci. 2019;23:7217–23.
Liu S, Suo J, Wang C, Sun X, Wang D, He L, et al. Prognostic significance of low miR-144 expression in gastric cancer. Cancer Biomarkers: Sect A Dis Mark. 2017;20:547–52.
Li J, Sun P, Yue Z, Zhang D, You K, Wang J. miR-144-3p induces cell cycle arrest and apoptosis in pancreatic cancer cells by targeting proline-rich protein 11 expression via the mitogen-activated protein kinase signaling pathway. DNA Cell Biol. 2017;36:619–26.
Liu C, Yang Z, Deng Z, Zhou Y, Gong Q, Zhao R, et al. Downregulated miR-144-3p contributes to progression of lung adenocarcinoma through elevating the expression of EZH2. Cancer Med. 2018;7:5554–66.
Cheng ZX, Song YX, Wang ZY, Wang Y, Dong Y. miR-144-3p serves as a tumor suppressor by targeting FZD7 and predicts the prognosis of human glioblastoma. Eur Rev Med Pharmacol Sci. 2017;21:4079–86.
Chen B, Xu P, Wang J, Zhang C. The role of MiRNA in polycystic ovary syndrome (PCOS). Gene. 2019;706:91–96.
Ilie IR, Georgescu CE. Polycystic ovary syndrome-epigenetic mechanisms and aberrant MicroRNA. Adv Clin Chem. 2015;71:25–45.
Li Y, Fang Y, Liu Y, Yang X. MicroRNAs in ovarian function and disorders. J Ovarian Res. 2015;8:51.
Toloubeydokhti T, Bukulmez O, Chegini N. Potential regulatory functions of microRNAs in the ovary. Sem Reprod Med. 2008;26:469–78.
Stubbs SA, Stark J, Dilworth SM, Franks S, Hardy K. Abnormal preantral folliculogenesis in polycystic ovaries is associated with increased granulosa cell division. J Clin Endocrinol Metab. 2007;92:4418–26.
Kampinga HH, Hageman J, Vos MJ, Kubota H, Tanguay RM, Bruford EA, et al. Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones. 2009;14:105–11.
Lackie RE, Maciejewski A, Ostapchenko VG, Marques-Lopes J, Choy WY, Duennwald ML, et al. The Hsp70/Hsp90 chaperone machinery in neurodegenerative diseases. Front Neurosci. 2017;11:254.
Witkin SS, Kanninen TT, Sisti G. The role of Hsp70 in the regulation of autophagy in gametogenesis, pregnancy, and parturition. Adv Anat Embryol Cell Biol. 2017;222:117–27.
Ruan X, Dai Y. Study on chronic low-grade inflammation and influential factors of polycystic ovary syndrome. Med Princ Pract. 2009;18:118–22.
Molvarec A, Rigo J Jr, Lazar L, Balogh K, Mako V, Cervenak L, et al. Increased serum heat-shock protein 70 levels reflect systemic inflammation, oxidative stress and hepatocellular injury in preeclampsia. Cell Stress Chaperones. 2009;14:151–9.
Peracoli JC, Bannwart-Castro CF, Romao M, Weel IC, Ribeiro VR, Borges VT, et al. High levels of heat shock protein 70 are associated with pro-inflammatory cytokines and may differentiate early- from late-onset preeclampsia. J Reprod Immun. 2013;100:129–34.
Gao H, Meng J, Xu M, Zhang S, Ghose B, Liu J, et al. Serum heat shock protein 70 concentration in relation to polycystic ovary syndrome in a non-obese chinese population. PloS ONE. 2013;8:e67727.
Wu G, Hu X, Ding J, Yang J. Abnormal expression of HSP70 may contribute to PCOS pathology. J Ovarian Res. 2019;12:74.
Funding
This work was supported by National Key Research and Development Program of China [Grant No. 2018YFC1002804 and No. 2016YFC1000600], National Natural Science Foundation of China [Grant No. 81571513, No. 81771662, No. 81771618 and No. 81801540], and the Major Technological Innovation Projects in Hubei Province [Grant No. 2017ACA101].
Author information
Authors and Affiliations
Contributions
JY and YZ designed the work; BQ and QZ performed the experiments; QM, TY, and XL collected the data and did the analysis; YC contributed essential reagents or tools; BQ drafted the manuscript; All of the authors approved the final version.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Qu, B., Zhao, Q., Ma, Q. et al. Overexpression of miR-144-3p alleviates polycystic ovaries syndrome through targeting expression of HSP-70. Gene Ther 29, 217–226 (2022). https://doi.org/10.1038/s41434-020-00191-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41434-020-00191-0
This article is cited by
-
Long non-coding RNA SNHG4 aggravates cigarette smoke-induced COPD by regulating miR-144-3p/EZH2 axis
BMC Pulmonary Medicine (2023)
-
MicroRNA-144-3p protects against chemotherapy-induced apoptosis of ovarian granulosa cells and activation of primordial follicles by targeting MAP3K9
European Journal of Medical Research (2023)
-
microRNA-194 is increased in polycystic ovary syndrome granulosa cell and induce KGN cells apoptosis by direct targeting heparin-binding EGF-like growth factor
Reproductive Biology and Endocrinology (2021)
-
The abnormal level of HSP70 is related to Treg/Th17 imbalance in PCOS patients
Journal of Ovarian Research (2021)