Abstract
Purpose
To evaluate the mid-term clinical results and the safety aspects of the Hydrus® Microstent (Ivantis, Inc, Irvine, CA) in a real-life setting.
Design
Retrospective case series.
Methods
Hydrus® Microstent was implanted in phakic eyes (88 eyes, 87.1%) and in pseudophakic eyes (13 eyes, 12.9%), respectively. Mean follow-up time was 16 ± 9 months with 27 eyes having a follow-up time of more than 24 months.
Main outcome measures
The primary endpoint was reduction in IOP compared to baseline. Target IOP levels were set at ≤20 mmHg, ≤18 mmHg and ≤15 mmHg. Kaplan–Meier survival was defined as a reduction in IOP of ≥20% compared to baseline. Secondary endpoints were reduction in number of glaucoma medications and safety assessments addressing visual acuity, adverse events, re-surgery rate and identification of factors that made the implantation more difficult.
Result
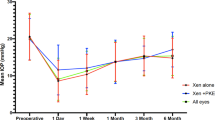
101 eyes underwent Hydrus® implantation. The mean preoperative IOP was 21.60 mmHg (SD 6.6) on 2.18 (SD 1.3) medications. After a mean follow up time of 16 months, the mean IOP was reduced to 14.61 ± 3.7 mmHg on 1.12 (SD 1.1) medication classes (p < 0.001). Mean decrease in IOP was 26.7%. Analysis of the target IOP levels showed that in 29%, 34% and 35% of cases an IOP of ≤15 mmHg, ≤18 mmHg and ≤20 mmHg respectively could be achieved. BCVA improved from 0.56 ± 0.3 at baseline to 0.85 ± 0.3 more than 24 months after surgery (p < 0.001). The rate of re-operation was low at <3%. Adverse events occurred in 4 eyes (<4%).
Conclusion
This study underlines the effectiveness and the safety of the Hydrus® Microstent in an elective setting, but it also demonstrates certain limits and risk factors of this procedure.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 18 print issues and online access
$259.00 per year
only $14.39 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The data that support the findings of this study are available on request from the corresponding author V. Prokosch. The data are not publicly available due to privacy restrictions.
References
Heijl A, Leske MC, Bengtsson B, Hyman L, Bengtsson B, Hussein M, et al. Reduction of Intraocular Pressure and Glaucoma Progression Results From the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002;120:1268–79.
Brandão LM, Grieshaber MC. Update on Minimally Invasive Glaucoma Surgery (MIGS) and New Implants. J Ophthalmol. 2013;705915. https://doi.org/10.1155/2013/705915
Richter GM, Coleman AL. Minimally invasive glaucoma surgery: current status and future prospects. Clin Ophtalmol. 2016;10:189–206.
Samuelson TW, Katz LJ, Wells JM, Duh Y-J, Giamporcaro JE, US iStent Study Group. Randomized evaluation of the trabecular micro-bypass stent with phacoemulsification in patients with glaucoma and cataract. Ophthalmology. 2011;118:459–67.
Vold S, Ahmed IIK, Craven ER, Mattox C, Stamper R, Packer M, et al. Two-Year COMPASS Trial Results: Supraciliary Microstenting with Phacoemulsification in Patients with Open-Angle Glaucoma and Cataracts. Ophthalmology. 2016;123:2103–12.
Lewis RA. Ab interno approach to the subconjunctival space using a collagen glaucoma stent. J Cataract Refract Surg. 2014;40:1301–6.
European Glaucoma Society. Terminology and guidelines for glaucoma. 5th ed. Publicomm; 2021, https://www.eugs.org/eng/guidelines.asp.
Johnstone MA, Sagheb H, Ahmed IIK, Samuelson TW, Schieber AT, Toris CB. Effects of a Schlemm canal scaffold on collector channel ostia in human anterior segments. Exper Eye Res. 2014;119:70–6.
Ivantis. Hydrus® Microstent Instructions for Use. Ivantis, Inc.; 2018. https://www.ivantisinc.com/isi/, Instruction for Use Hydrus Microstent.
Pfeiffer N, Garcia-Feijoo J, Martinez-de-la-Casa JM, Larrosa JM, Fea A, Lemij H, et al. A Randomized Trial of a Schlemm’s Canal Microstent with Phacoemulsification for Reducing Intraocular Pressure in Open-Angle Glaucoma. Ophthalmology. 2015;122:1283–93.
Samuelson TW, Chang DF, Marquis R, Flowers B, Lim KS, Ahmed IIK, et al. A Schlemm Canal Microstent for Intraocular Pressure Reduction in Primary Open-Angle Glaucoma and Cataract: The HORIZON Study. Ophthalmology. 2018;126:29–37.
Samet S, Ong JA, Ahmed IIK. Hydrus microstent implantation for surgical management of glaucoma: a review of design, efficacy and safety. Eye Vis. 2019;6:32.
U.S Food and Drug Administration (FDA). Premarket Approval Application (PMA) Number: P170034: FDA Summary of Safety and Effectiveness Data. 2018. https://www.fda.gov/medical-devices/premarket-submissions-selecting-and-preparing-correct-submission/premarket-approval-pma
Tarek M, Shaarawy MBS, Franz G. World Glaucoma Association. Guidelines on Design & Reporting of Glaucoma Surgical Trials. Kugler Publications; 2018, https://wga.one/wpfd_file/guidelines-on-design-reporting-glaucoma-trials/.
Fea AM, Belda JI, Rękas M, Jünemann A, Chang L, Pablo L, et al. Prospective unmasked randomized evaluation of the iStent inject® versus two ocular hypotensive agents in patients with primary open-angle glaucoma. Clin Ophtalmol. 2014;8:875–82.
Craven ER, Katz LJ, Wells JM, Giamporcaro JE. Cataract surgery with trabecular micro-bypass stent implantation in patients with mild-to-moderate open-angle glaucoma and cataract: two-year follow-up. J Cataract Refract Surg. 2012;38:1339–45.
Voskanyan L, García-Feijoó J, Belda JI, Fea A, Jünemann A, Baudouin C. Prospective, Unmasked Evaluation of the iStent® Inject System for Open-Angle Glaucoma: Synergy Trial. Adv Therapy. 2014;31:189–201.
Fea AM, Ahmed II, Lavia C, Mittica P, Consolandi G, Motolese I, et al. Hydrus microstent compared to selective laser trabeculoplasty in primary open angle glaucoma: one year results. Clin Exper Ophtalmol. 2017;45:120–7.
Gandolfi SA, Ungaro N, Ghirardini S, Tardini MG, Mora P. Comparison of Surgical Outcomes between Canaloplasty and Schlemm’s Canal Scaffold at 24 Months’ Follow-Up. J Ophthalmol. 2016;3410469. https://doi.org/10.1155/2016/3410469
Pfeiffer N. Moderne medikamentöse Glaukomtherapie. Dtsch Arztebl International. 1998;95:(S. 95).
Fea AM, Rekas M, Au L. Evaluation of a Schlemm canal scaffold microstent combined with phacoemulsification in routine clinical practice: Two-year multicenter study. J Cataract Refract Surg. 2017;43:886–91.
Kim DD, Doyle JW, Smith MF. Intraocular pressure reduction following phacoemulsification cataract extraction with posterior chamber lens implantation in glaucoma patients. Ophthalmic Surg Lasers Imaging Retina. 1999;30:37–40.
Shingleton BJ, Gamell LS, O’Donoghue MW, Baylus SL, King R. Long-term changes in intraocular pressure after clear corneal phacoemulsification: Normal patients versus glaucoma suspect and glaucoma patients. J Cataract Refract Surg. 1999;25:885–90.
Shingleton BJ, Pasternack JJ, Hung JW, O’Donoghue MW. Three and Five Year Changes in Intraocular Pressures After Clear Corneal Phacoemulsification in Open Angle Glaucoma Patients, Glaucoma Suspects, and Normal Patients. J Glaucoma. 2006;15:494–8.
Poley BJ, Lindstrom RL, Samuelson TW, Schulze R Jr. Intraocular pressure reduction after phacoemulsification with intraocular lens implantation in glaucomatous and nonglaucomatous eyes: Evaluation of a causal relationship between the natural lens and open-angle glaucoma. J Cataract Refract Surg. 2009;35:1946–55.
Funding
Ivantis, Inc. provided the Hydrus® Microstent devices used free of charge. This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
DK, KL and VP conceived and designed the analysis and wrote the paper. DK and LL-B performed the analysis. VP, UV and NP contributed data.
Corresponding author
Ethics declarations
Competing interests
The data from this study were made available to Ivantis Inc. before the publication. However, no restrictions from the manufacturer were exerted nor accepted concerning the manuscript body.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kiramira, D., Voßmerbäumer, U., Pfeiffer, N. et al. Mid-term real world outcomes of the Hydrus® Microstent in open angle glaucoma. Eye (2024). https://doi.org/10.1038/s41433-023-02920-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41433-023-02920-2