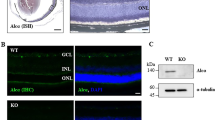
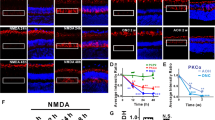
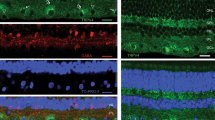
Abstract
Amacrine cells (ACs) are the most structurally and functionally diverse neuron type in the retina. Different ACs have distinct functions, such as neuropeptide secretion and inhibitory connection. Vasoactive intestinal peptide (VIP) -ergic -ACs are retina gamma-aminobutyric acid (GABA) -ergic -ACs that were discovered long ago. They secrete VIP and form connections with bipolar cells (BCs), other ACs, and retinal ganglion cells (RGCs). They have a specific structure, density, distribution, and function. They play an important role in myopia, light stimulated responses, retinal vascular disease and other ocular diseases. Their significance in the study of refractive development and disease is increasing daily. However, a systematic review of the structure and function of retinal VIP-ACs is lacking. We discussed the detailed characteristics of VIP-ACs from every aspect across species and providing systematic knowledge base for future studies. Our review led to the main conclusion that retinal VIP-ACs develop early, and although their morphology and distribution across species are not the same, they have similar functions in a wide range of ocular diseases based on their function of secreting neuropeptides and forming inhibitory connections with other cells.
摘要
无长突细胞(ACs)是视网膜中结构和功能最多样的神经元类型。不同的ACs具有不同的功能, 如神经肽分泌和抑制连接等。血管活性肠肽(VIP)能AC是较久远发现的视网膜γ-氨基丁酸(GABA)能ACs, 分泌VIP并与双极细胞(BC)、其他 AC和视网膜神经节细胞(RGC)形成连接。它们具有特定的结构、密度、分布和功能。它们在近视、光刺激反应、视网膜血管疾病和其他眼部疾病的发病机制中起重要作用。它们在屈光发育和疾病研究中的作用及意义越来越大。然而, 目前关于视网膜VIP-ACs 的结构和功能缺乏系统综述。我们从不同物种的各个方面详细讨论了VIP-AC的特点, 为今后的研究提供了系统的知识基础。本综述得出的主要结论是, 视网膜 VIP-AC发育较早, 尽管它们的形态和分布在物种之间不同, 但基于其分泌神经肽和与其他细胞形成抑制性连接的功能在各种眼部疾病中具有相似的功能。
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 18 print issues and online access
$259.00 per year
only $14.39 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All relevant data are included in this manuscript.
References
Yan W, Laboulaye MA, Tran NM, Whitney IE, Benhar I, Sanes JR. Mouse retinal cell atlas: molecular identification of over sixty amacrine cell types. J Neurosci. 2020;40:5177–95.
Lammerding-Koppel M, Thier P, Koehler W. Morphology and mosaics of VIP-like immunoreactive neurons in the retina of the rhesus monkey. J Comp Neurol. 1991;312:251–63.
Cepko C. Intrinsically different retinal progenitor cells produce specific types of progeny. Nat Rev Neurosci. 2014;15:615–27.
Giunta S, Castorina A, Bucolo C, Magro G, Drago F, D’Agata V. Early changes in pituitary adenylate cyclase-activating peptide, vasoactive intestinal peptide and related receptors expression in retina of streptozotocin-induced diabetic rats. Peptides. 2012;37:32–39.
Zhao F, Li Q, Chen W, Zhu H, Zhou D, Reinach PS, et al. Dysfunction of VIPR2 leads to myopia in humans and mice. J Med Genet. 2022;59:88–100.
Shoge K, Mishima HK, Saitoh T, Ishihara K, Tamura Y, Shiomi H, et al. Protective effects of vasoactive intestinal peptide against delayed glutamate neurotoxicity in cultured retina. Brain Res. 1998;809:127–36.
VERUKI ML, YEH HH. Vasoactive intestinal polypeptide modulates GABAA receptor function through activation of cyclic AMP. Vis Neurosci. 1994;11:899–908.
Akrouh A, Kerschensteiner D. Morphology and function of three VIP-expressing amacrine cell types in the mouse retina. J Neurophysiol. 2015;114:2431–8.
Said SI, Mutt V. Polypeptide with broad biological activity: isolation from small intestine. Science. 1970;169:1217–8.
Loren I, Tornqvist K, Alumets J. VIP (Vasoactive Intestinal Polypeptide)-immunoreactive Neurons in the Retina of the Rat. Cell Tissue Res. 1980;210:167–70.
Casini G, Brecha NC. Colocalization of vasoactive intestinal polypeptide and GABA immunoreactivities in a population of wide-field amacrine cells in the rabbit retina. Vis Neurosci. 1992;8:373–8.
Casini G, Rickman DW, Brecha NC. Expression of the gamma-aminobutyric acid (GABA) plasma membrane transporter-1 in monkey and human retina. Investig Ophthalmol Vis Sci. 2006;47:1682–90.
Kondo H, Kuramoto H, Wainer B, Yanaihara N. Discrete distribution of cholinergic and vasoactive intestinal polypeptidergic amacrine cells in the rat retina. Neurosci Lett. 1985;54:213–8.
Perez de Sevilla Muller L. Localisation of neuronal nitric oxide synthase-immunoreactivity in rat and rabbit retinas. Exp Brain Res. 1995;4:207–17.
Menger N, Seidenbecher CI, Gundelfinger ED, Kreutz MR. The cytoskeleton-associated neuronal calcium-binding protein caldendrin is expressed in a subset of amacrine, bipolar and ganglion cells of the rat retina. Cell Tissue Res. 1999;298:21–32.
Mikkelsen JD, Larsen JNB, Fahrenkrug J, Moller M. Peptide histidine-isoleucine (PHI)-immunoreactive amacrine cells in the retina of the rat. Neurosci Lett. 1987;79:281–5.
Nishizawa Mikio, Hayakawa Yumiko, Yanaihara N, Okamoto H. Nucleotide sequence divergence and functional constraint in VIP precursor mRNA. FEBS Lett. 1985;183:55–59.
Miyata A, Arimura A, Dahl R, Minamino N, Uehara A, Jiang L, et al. Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells. Biochem Biophys Res Commun. 1989;164:567–74.
Seki T, Shioda S, Nakai Y, Arimura A, Koide R. Distribution and ultrastructural localization of pituitary adenylate cyclase-activating polypeptide (PACAP) and its receptor in the rat retina. Ann N Y Acad Sci. 1998;865:408–11.
Horsburgh GM, Sefton AJ. Cellular degeneration and synaptogenesis in the developing retina of the rat. J Comp Neurol. 1987;263:553–66.
Herbst H, Thier P. Different effects of visual deprivation on vasoactive intestinal polypeptide (VIP)-containing cells in the retinas of juvenile and adult rats. Exp Brain Res. 1996;111:345–55.
Terubayashi H, Okamura H, Fujisawa H, Itoi M, Yanaihara N, Ibata Y. Postnatal-development of vasoactive intestinal polypeptide imminoreactive amacrine cells in the rat retina. Neurosci Lett. 1982;33:259–64.
Casini G, Molnar M, Brecha NC. Vasoactive intestinal polypeptide/peptide histidine isoleucine messenger RNA in the rat retina: adult distribution and developmental expression. Neuroscience. 1994;58:657–67.
Lee S, Meyer A, Schubert T, Hüser L, Dedek K, Haverkamp S. Morphology and connectivity of the small bistratified A8 amacrine cell in the mouse retina. J Comp Neurol. 2015;523:1529–47.
Perez de Sevilla Muller L, Solomon A, Sheets K, Hapukino H, Rodriguez AR, Brecha NC. Multiple cell types form the VIP amacrine cell population. J Comp Neurol. 2019;527:133–58.
Park SJ, Borghuis BG, Rahmani P, Zeng Q, Kim IJ, Demb JB. Function and circuitry of VIP+ interneurons in the mouse retina. J Neurosci. 2015;35:10685–700.
Casini G, Brecha NC. Vasoactive intestinal polypeptide-containning cells in the rabbit retina-immunohistochemical localization and quantitative analysis. J Comp Neurol. 1991;305:313–27.
Zhu Y, Xu J, Hauswirth WW, DeVries SH. Genetically targeted binary labeling of retinal neurons. J Neurosci. 2014;34:7845–61.
Lee EJ, Park SH, Kim IB, Kang WS, Oh SJ, Chun MH. Light- and electron-microscopic analysis of vasoactive intestinal polypeptide-immunoreactive amacrine cells in the guinea pig retina. J Comp Neurol. 2002;445:325–35.
Kiyama H, Katayamakumoi Y, Kimmel J, Steinbusch H, Powell JF, Smith AD, et al. Three dimensional analysis of retinal neuropeptides and amine in the chick. Brain Res Bull. 1985;15:155–65.
Terubayashi H, Tsuto T, Fukui K, Obata HL, Okamura H, Fujisawa H, et al. VIP (vasoactive intestinal polypeptide) -like immunoreactive amacrine cells in the retina of the rat. Exp eye Res. 1983;36:743–9.
Ekman R, Tornqvist K. Glucagon and VIP in the retina. Invest Ophthalmol Vis Sci. 1985;26:1405–9.
Tornqvist K, Uddman R, Sundler F, Ehinger B. Somatostatin and VIP neurons in the retina of different species. Histochemistry. 1982;76:137–52.
Eriksen EF, Larsson LI. Neuropeptides in the retina: evidence for differential topographical localization. Peptides. 1981;2:153–7.
Sagar SM. Vasoactive intestinal polypeptide (VIP) immunohistochemistry in the rabbit retina. Brain Res. 1987;426:157–63.
Tornqvist K, Ehinger B. Peptide immunoreactive neurons in the human retina. Invest Ophthalmol Vis Sci. 1988;29:680–6.
Li HB, Lam DM. Localization of neuropeptide-immunoreactive neurons in the human retina. Brain Res. 1990;522:30–36.
Zhang X, Wang X, Wang S, Peng W, Ullah R, Fu J, et al. Trilogy development of proopiomelanocortin neurons from embryonic to adult stages in the mice retina. Front Cell Dev Biol. 2021;9:718851.
Munteanu T, Noronha KJ, Leung AC, Pan S, Lucas JA, Schmidt TM. Light-dependent pathways for dopaminergic amacrine cell development and function. eLife. 2018;7:e39866.
Keeley PW, Whitney IE, Madsen NR, St John AJ, Borhanian S, Leong SA, et al. Independent genomic control of neuronal number across retinal cell types. Dev Cell. 2014;30:103–9.
Menger N, Pow DV, Wassle H. Glycinergic amacrine cells of the rat retina. J Comp Neurol. 1998;401:34–46.
Masland RH, Mills JW, Hayden SA. Acetylcholine-synthesizing amacrine cells: identification and selective staining by using radioautography and fluorescent markers. Proc R Soc Ser B-Biol Sci. 1984;223:79–100.
Sandell JH, Masland RH. A system of indoleamine-accumulating neurons in the rabbit retina. J Neurosci. 1986;6:3331–47.
Vaney DI, Young HM. GABA-like immunoreactivity in NADPH-diaphorase amacrine cells of the rabbit retina. Brain Res. 1988;474:380–5.
Rickman DW, Blanks JC, Brecha NC. Somatostatin-immunoreactive neurons in the adult rabbit retina. J Comp Neurol. 1996;365:491–503.
Wassle H, Riemann HJ. Mosaic of nerve-cells in the mammalian retina. Proc R Soc Ser B-Biol Sci. 1978;200:441–61.
Jensen RJ. Effects of vasoactive intestinal peptide on ganglion cells in the rabbit retina. Vis Neurosci. 1993;10:181–9.
McGuire B, Stevens J, Sterling P. Microcircuitry of beta ganglion cells in cat retina. J Neurosci. 1986;6:907–18.
McGuire B, Stevens J, Sterling P. Microcircuitry of bipolar cells in cat retina. J Neurosci. 1984;4:2920–38.
Wassle H. Parallel processing in the mammalian retina. Nat Rev Neurosci. 2004;5:747–57.
Uddman R, Alumets J, Ehinger B, Håkanson R, Lorén I, Sundler F. Vasoactive intestinal peptide nerves in ocular and orbital structures of the cat. Invest Ophthalmol Vis Sci. 1980;19:878–85.
Bleckert A, Zhang C, Turner MH, Koren D, Berson DM, Park SJH, et al. GABA release selectively regulates synapse development at distinct inputs on direction-selective retinal ganglion cells. Proc Natl Acad Sci USA. 2018;115:E12083–E12090.
Muller LPDS, Santos JDL, Brecha N. Modulation of VIP-1 amacrine cell coupling by dopamine in the mouse retina. IOVS. 2019;60:543.
Koistinaho J, Sagar SM. Light-induced c-f&s expression in amacrine cells in the rabbit retina. Mol Brain Res. 1995;29:53–63.
Wiesei TN, Raviola E. Myopia and eye enlargement after neonatal lid fusion in monkeys. Nature. 1977;266:66–68.
Tian L, Guo YT, Ying M, Liu YC, Li X, Wang Y. Co-existence of myopia and amblyopia in a guinea pig model with monocular form deprivation. Ann Transl Med. 2021;9:110.
McGlinn AM, Baldwin DA, Tobias JW, Budak MT, Khurana TS, Stone RA. Form-deprivation myopia in chick induces limited changes in retinal gene expression. Invest Ophthalmol Vis Sci. 2007;48:3430–6.
Cakmak AI, Basmak H, Gursoy H, Ozkurt M, Yildirim N, Erkasap N, et al. Vasoactive intestinal peptide, a promising agent for myopia? Int J Ophthalmol. 2017;10:211–6.
Stone RA, Laties AM, Raviola E, Wiesel TN. Increase in retinal vasoactive intestinal polypeptide after eyelid fusion in primates (retina/myopia). Proc Nat Acad Sci. 1988;85:257–60.
Tkatchenko AV, Walsh PA, Tkatchenko TV, Gustincich S, Raviola E. Form deprivation modulates retinal neurogenesis in primate experimental myopia. Proc Natl Acad Sci USA. 2006;103:4681–6.
Wang P-B, Wang H, Liu S-Z, Jiang J-J. Effect of vasoactive intestinal peptide receptor antagonist VIPhybrid on the development of form deprivation myopia in chicks. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2008;33:669–75.
Mao J-F, Liu S-Z. Mechanism of the DL-alpha-aminoadipic acid inhibitory effect on form-deprived myopia in guinea pig. Int J Ophthalmol. 2013;6:19–22.
Pickett Seltner RL, Stell WK. The effect of vasoactive intestinal peptide on development of form deprivation myopia in the chick: a pharmacological and immunocytochemical study. Vis Res. 1995;35:1265–70.
Mathis U, Schaeffel F. Glucagon-related peptides in the mouse retina and the effects of deprivation of form vision. Graefes Arch Clin Exp Ophthalmol. 2007;245:267–75.
Brand C, Burkhardt E, Schaeffel F, Choi JW, Feldkaemper MP. Regulation of Egr-1, VIP, and Shh mRNA and Egr-1 protein in the mouse retina by light and image quality. Mol Vis. 2005;11:309–20.
He L, Frost MR, Siegwart JT, Norton TT. Gene expression signatures in tree shrew choroid during lens-induced myopia and recovery. Exp Eye Res. 2014;123:56–71.
Werner G, Mitterauer BJ. Neuromodulatory systems. Front Neural Circuits. 2013;7:36.
Zhong X, Ge J, Smith EL 3rd, Stell WK. Image defocus modulates activity of bipolar and amacrine cells in macaque retina. Invest Ophthalmol Vis Sci. 2004;45:2065–74.
Lakk M, Szabó B, Völgyi B, Gábriel R, Dénes V. Development-related splicing regulates pituitary adenylate cyclase-activating polypeptide (PACAP) receptors in the retina. Invest Ophthalmol Vis Sci. 2012;53:7825–32.
Shi Y, Gong B, Chen L, Zuo X, Liu X, Tam POS, et al. A genome-wide meta-analysis identifies two novel loci associated with high myopia in the Han Chinese population. Hum Mol Genet. 2013;22:2325–33.
Yiu WC, Yap MK, Fung WY, Ng PW, Yip SP. Genetic susceptibility to refractive error: association of vasoactive intestinal peptide receptor 2 (VIPR2) with high myopia in Chinese. PLoS One. 2013;8:e61805.
Naiglin L, Gazagne C, Dallongeville F, Thalamas C, Idder A, Rascol O, et al. A genome wide scan for familial high myopia suggests a novel locus on chromosome 7q36. J Med Genet. 2002;39:118–24.
Liu S-Z, Wang H, Jiang J-J, Wang P-B, Wu X-Y, Tan X-P, et al. Dynamic expression of VIPR2 in form deprivation myopia. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2005;30:456–9.
Leung KH, Luo S, Kwarteng R, Chen S-G, Yap MKH, Huang C-L, et al. The myopia susceptibility locus vasoactive intestinal peptide receptor 2 (VIPR2) contains variants with opposite effects. Sci Rep. 2019;9:18165.
Vaudry D, Falluel-Morel A, Bourgault S, Basille M, Burel D, Wurtz O, et al. Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol Rev. 2009;61:283–357.
Harmar AJ, Marston HM. The VPAC2 receptor is essential for circadian function in the mouse suprachiasmatic nuclei. Cell. 2000;109:497–508.
Nickla DL. Ocular diurnal rhythms and eye growth regulation: where we are 50 years after Lauber. Exp Eye Res. 2013;114:25–34.
De-Miguel FF, Trueta C. Synaptic and extrasynaptic secretion of serotonin. Cell Mol Neurobiol. 2005;25:297–312.
Puopolo M, Hochstetler SE, Gustincich S, Wightman RM, Raviola E. Extrasynaptic release of dopamine in a retinal neuron: activity dependence and transmitter modulation. Neuron. 2001;30:211–25.
Trueta C, Mendez B, De-Miguel FF. Somatic exocytosis of serotonin mediated by L-type calcium channels in cultured leech neurones. J Physiol. 2003;547:405–16.
Trueta C, Sánchez-Armass S, Morales M, De-Miguel F. Calcium-induced calcium release contributes to somatic secretion of serotonin in leech Retzius neurons. J Neurobiol. 2004;61:309–16.
Delgado M, Reduta A, Sharma V, Ganea D. VIP/PACAP oppositely affects immature and mature dendritic cell expression of CD80/CD86 and the stimulatory activity for CD4(+) T cells. J Leukoc Biol. 2004;75:1122–30.
Abad C, Jayaram B, Becquet L, Wang Y, O’Dorisio MS, Waschek JA, et al. VPAC1 receptor (Vipr1)-deficient mice exhibit ameliorated experimental autoimmune encephalomyelitis, with specific deficits in the effector stage. J Neuroinflammation. 2017;14:1–14.
Olson KE, Kosloski-Bilek LM, Anderson KM, Diggs BJ, Clark BE, Gledhill JM, et al. Selective VIP receptor agonists facilitate immune transformation for dopaminergic neuroprotection in MPTP-intoxicated mice. J Neurosci. 2015;35:16463–78.
Shi H, Carion TW, Jiang Y, Steinle JJ, Berger EA. VIP protects human retinal microvascular endothelial cells against high glucose-induced increases in TNF-alpha and enhances RvD1. Prostaglandins Other Lipid Mediat. 2016;123:28–32.
Scuderi S, D’Amico AG, Castorina A, Imbesi R, Carnazza ML, D’Agata V. Ameliorative effect of PACAP and VIP against increased permeability in a model of outer blood retinal barrier dysfunction. Peptides. 2013;39:119–24.
Castorina A, Giunta S, Mazzone V, Cardile V, D’Agata V. Effects of PACAP and VIP on hyperglycemia-induced proliferation in murine microvascular endothelial cells. Peptides. 2010;31:2276–83.
Troger J, Neyer S, Heufler C, Huemer H, Schmid E, Griesser U, et al. Substance P acid vasoactive intestinal polypeptide in the streptozotocin-induced diabetic rat retina. Investig Ophthalmol Vis Sci. 2001;42:1045–50.
Ramsey D, Ripps H, Qian H. Streptozotocin-induced diabetes modulates GABA receptor activity of rat retinal neurons. Exp Eye Res. 2007;85:413–22.
Tuncel N, Basmak H, Uzuner K, Tuncel M, Altiokka G, Zaimoglu V, et al. Protection of rat retina from ischemia-reperfusion injury by vasoactive intestinal peptide (VIP): the effect of VIP on lipid peroxidation and antioxidant enzyme activity of retina and choroid. Ann N Y Acad Sci. 1996;805:489–98.
Reiner A, Fitzgerald M, Del Mar N, Li C. Neural control of choroidal blood flow. Prog retinal eye Res. 2018;64:96–130.
Maugeri G, D’Amico AG, Rasà DM, Saccone S, Federico C, Cavallaro S, et al. PACAP and VIP regulate hypoxia-inducible factors in neuroblastoma cells exposed to hypoxia. Neuropeptides. 2018;69:84–91.
Maugeri G, D’Amico AG, Saccone S, Federico C, Cavallaro S, D’Agata V. PACAP and VIP inhibit HIF-1α-mediated VEGF expression in a model of diabetic macular edema. J Cell Physiol. 2017;232:1209–15.
D’Amico AG, Maugeri G, Rasa DM, La Cognata V, Saccone S, Federico C, et al. NAP counteracts hyperglycemia/hypoxia induced retinal pigment epithelial barrier breakdown through modulation of HIFs and VEGF expression. J Cell Physiol. 2018;233:1120–8.
Scuderi S, D’Amico AG, Castorina A, Federico C, Marrazzo G, Drago F, et al. Davunetide (NAP) protects the retina against early diabetic injury by reducing apoptotic death. J Mol Neurosci. 2014;54:395–404.
Lasater EM, Watling KJ, Dowling JE. Vasoactive intestinal peptide alters membrane potential and cyclic nucleotide levels in retinal horizontal cells. Science. 1983;221:1070–2.
Kozorovitskiy Y, Peixoto R, Wang W, Saunders A, Sabatini BL. Neuromodulation of excitatory synaptogenesis in striatal development. eLife. 2015;4:1–18.
Huganir RL, Greengard P. Regulation of neurotransmitter receptor desensitization by protein phosphorylation. Neuron. 1990;5:555–67.
Browning MD, Endo S, Smith GB, Dudek EM, Olsen RW. Phosphorylation of the GABAA receptor by cAMP-dependent protein kinase and by protein kinase C: analysis of the substrate domain. Neurochem Res. 1993;18:95–100.
VERUKI ML, YEH HH. Vasoactive intestinal polypeptide modulates GABA receptor function in bipolar cells and ganglion cells of the rat retina. J Neurophysiol. 1992;67:791–7.
Grigorenko EV, Yeh HH. Expression profiling of GABAA receptor β-subunits in the rat retina. Vis Neurosci. 1994;11:379–87.
Morita K, Sakakibara A, Kitayama S, Kumagai K, Tanne K, Dohi T. Pituitary adenylate cyclase-activating polypeptide induces a sustained increase in intracellular free Ca2+ concentration and catecholamine release by activating Ca2+ influx via receptor-stimulated Ca2+ entry, independent of store-operated Ca2+ channels, and voltage-dependent Ca2+ channels in bovine adrenal medullary chromaffin cells. J Pharmacol Exp Therapeutics. 2002;302:972–82.
Hedlund B, Dufy B, Barker L. Vasoactive intestinal polypeptide alters GH3/B6 pituitary cell excitability. Pflug Arch Eur J Physiol. 1988;411:173–9.
Goldsmith BA, Abrams TW. cAMP modulates multiple K+ currents, increasing spike duration and excitability in Aplysia sensory neurons. Proc Natl Acad Sci USA. 1992;89:11481–5.
Pachter JA, Lam DM-K. Interactions between vasoactive intestinal peptide and dopamine in the rabbit retina: stimulation of a common adenylate cyclase. J Neurochem. 1986;46:257–64.
Falktoft B, Georg B, Fahrenkrug J. Signaling pathways in PACAP regulation of VIP gene expression in human neuroblastoma cells. Neuropeptides. 2009;43:387–96.
Kaiser PK, Lipton SA. VIP-mediated increase in cAMP prevents tetrodotoxin-lnduced retinal ganglion cell death in vitro. Neuron. 1990;5:373–81.
Brenneman D, Glazner G, Hill J, Hauser J, Davidson A, Gozes I. VIP neurotrophism in the central nervous system: multiple effectors and identification of a femtomolar-acting neuroprotective peptide. Ann N Y Acad Sci. 1998;865:207–12.
McCoy HM, Polcyn R, Banik NL, Haque A. Regulation of enolase activation to promote neural protection and regeneration in spinal cord injury. Neural Regen Res. 2023;18:1457–62.
Fu Z, Yan W, Chen CT, Nilsson AK, Bull E, Allen W, et al. Omega-3/Omega-6 long-chain fatty acid imbalance in phase I retinopathy of prematurity. Nutrients. 2022;14:1333.
Koh SW, Roberge FG. VIP modulation of cultured glial cells of the rat retina. Curr Eye Res. 1989;8:1207–10.
Fang XL, Zhang Q, Xue WW, Tao JH, Zou HD, Lin QR et al. Suppression of cAMP/PKA/CREB signaling ameliorates retinal injury in diabetic retinopathy. Kaohsiung J Med Sci. 2023;39:916–26.
Brenneman DE, Hill JM, Glazner GW, Gozes I, Phillips TW. Interleukin-1 alpha and vasoactive interstinal peptide: Enigmatic regulation of neuronal survival. Int J Dev Neurosci. 1995;13:187–200.
Festoff BW, Nelson PG, Brenneman DE. Prevention of activity-dependent neuronal death: vasoactive intestinal polypeptide stimulates astrocytes to secrete the thrombin-inhibiting neurotrophic serpin, protease nexin I. J Neurobiol. 1996;30:255–66.
Brenneman D, Gozes I. A femtomolar-acting neuroprotective peptide. J Clin Investig. 1996;97:2299–307.
Gozes I, Brenneman DE. A new concept in the pharmacology of neuroprotection. J Mol Neurosci. 2000;14:61–68.
Bassan M, Zamostiano R, Davidson A, Pinhasov A, Giladi E, Perl O, et al. Complete sequence of a novel protein containing a femtomolar-activity-dependent neuroprotective peptide. J Neurochem. 1999;72:1283–93.
Servoss SJ, Lee SJ, Lee SJ, Gozes I, Brenneman DE, Hill JM. IGF-I as a mediator of VIP/activity-dependent neurotrophic factor-stimulated embryonic growth. Endocrinology. 2001;142:3348–53.
Jiang X, McClellan SA, Barrett RP, Berger EA, Zhang Y, Hazlett LD. VIP and growth factors in the infected cornea. Invest Ophthalmol Vis Sci. 2011;52:6154–61.
Qin X, Sun X, Luo Z, Guan C, Zhang C. Affection of epidermal growth factor on VIP secretion and VIPR expression in airway epithelial cells. Hunan Yi Ke Da Xue Xue Bao. 1999;24:99–102.
Belokopytov M, Shulman S, Dubinsky G, Gozes I, Belkin M, Rosner M. Ameliorative effect of NAP on laser-induced retinal damage. Acta Ophthalmologica. 2011;89:E126–E131.
Zheng YP, Zeng H, She HN, Liu H, Sun NX. Expression of peptide NAP in rat retinal Muller cells prevents hypoxia-induced retinal injuries and promotes retinal neurons growth. Biomed Pharmacother. 2010;64:417–23.
Acknowledgements
We would like to thank the authors of the primary studies. We used Adobe Illustrator to create the figures in this article. We thank Biorender (https://app.biorender.com/) for providing the picturing materials.
Funding
We thank the following funding sources: The Strategic Priority Research Program of Chinese Academy of Sciences (XDA16040200); The Natural Science Foundation of Zhejiang Province (LZ19H120001); The National Nature Foundation Youth (Approval Number: 82201194); The National Nature Foundation (Approval Number: 82371084).
Author information
Authors and Affiliations
Contributions
X-HZ and X-YW wrote the manuscript, J-RZ, K-QC and UR prepared the references and revision of the manuscript, YS and T-JP arranged the manuscript and revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, X., Wang, X., Zhu, J. et al. Retinal VIP-amacrine cells: their development, structure, and function. Eye 38, 1065–1076 (2024). https://doi.org/10.1038/s41433-023-02844-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-023-02844-x