Abstract
Background
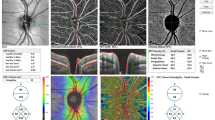
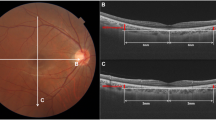
Eyes with peripapillary nerve fibre elevation (pNFE) may have a gap between the optic nerve papillary margin on colour fundus photography and Bruch’s membrane opening on cross-sectional optical coherence tomography (OCT). This study was conducted to evaluate the quantification of the height of pNFE in young healthy eyes and examine the relationship between pNFE height and axial length.
Methods
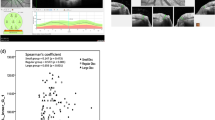
A prospective, observational, cross-sectional study was performed involving 117 right eyes. All participants (mean age 25.8 years) underwent comprehensive ophthalmologic examination involving axial length, fundus photography, and peripapillary and optic disc OCT. pNFE height was defined as the distance between the retinal surface plane and the upper edge of the pNFE in optic disc cross-sectional OCT images. Optic disc tilt was evaluated using a sine curve on retinal nerve fibre layer B-scan images. Parapapillary atrophy (PPA) area in colour fundus images was calculated using ImageJ and corrected using Bennett’s formula. We evaluated relationships between pNFE height, axial length, optic disc papillary tilt, and PPA area using Spearman’s correlation analysis.
Results
Sixty-five eyes had pNFE, with a mean pNFE height of 84.7 μm. pNFE height was significantly positively correlated with axial length (r = 0.32, p < 0.001), optic disc tilt (r = 0.25, p = 0.008), and PPA area (r = 0.27, p = 0.004).
Conclusions
pNFE is not rare in young healthy eyes. Eyes with higher pNFE had a longer axial length and larger optic disc tilt and PPA area.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 18 print issues and online access
$259.00 per year
only $14.39 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Data is available upon reasonable request.
References
Shields MB. Textbook of Glaucoma. Baltimore, MD: William & Wilkins; 1997.
Shaarawy TM, Sherwood MB, Hitchings RA, Crowston JG. Glaucoma: Medical Diagnosis and Therapy. Philadelphia, PA: Saunders Ltd; 2009.
The Japan Glaucoma Society Guidelines for Glaucoma. 3rd ed. Nippon Ganka Gakkai zasshi. 2012:116:3–46.
Iwase A, Suzuki Y, Araie M, Yamamoto T, Abe H, Shirato S, et al. The prevalence of primary open-angle glaucoma in Japanese: the Tajimi Study. Ophthalmology. 2004;111:1641–8.
Wang YX, Xu L, Yang H, Jonas JB. Prevalence of glaucoma in North China: the Beijing eye study. Am J Ophthalmol. 2010;150:917–24.
Kim CS, Seong GJ, Lee NH, Song KC, Namil Study Group, Korean Glaucoma Society. Prevalence of primary open-angle glaucoma in central South Korea the Namil study. Ophthalmology. 2011;118:1024–30.
Foster PJ, Oen FT, Machin D, Ng TP, Devereux JG, Johnson GJ, et al. The prevalence of glaucoma in Chinese residents of Singapore: a cross-sectional population survey of the Tanjong Pagar district. Arch Ophthalmol. 2000;118:1105–11.
Leske MC, Connell AM, Schachat AP, Hyman L. The Barbados eye study. Prevalence of open angle glaucoma. Arch Ophthalmol. 1994;112:821–9.
Bonomi L, Marchini G, Marraffa M, Bernardi P, De Franco I, Perfetti S, et al. Prevalence of glaucoma and intraocular pressure distribution in a defined population. The egna-Neumarkt study. Ophthalmology. 1998;105:209–15.
Buhrmann RR, Quigley HA, Barron Y, West SK, Oliva MS, Mmbaga BB. Prevalence of glaucoma in a rural East African population. Investig Ophthalmol Vis Sci. 2000;41:40–8.
Wensor MD, McCarty CA, Stanislavsky YL, Livingston PM, Taylor HR. The prevalence of glaucoma in the Melbourne visual impairment project. Ophthalmology. 1998;105:733–9.
Quigley HA, West SK, Rodriguez J, Munoz B, Klein R, Snyder R. The prevalence of glaucoma in a population-based study of Hispanic subjects: proyecto VER. Arch Ophthalmol. 2001;119:1819–26.
Rotchford AP, Johnson GJ. Glaucoma in Zulus: a population-based cross-sectional survey in a rural district in South Africa. Arch Ophthalmol. 2002;120:471–8.
Tielsch JM, Sommer A, Katz J, Royall RM, Quigley HA, Javitt J. Racial variations in the prevalence of primary open-angle glaucoma. The Baltimore Eye Survey. JAMA. 1991;266:369–74.
Mason RP, Kosoko O, Wilson MR, Martone JF, Cowan CL, Gear JC, et al. National survey of the prevalence and risk factors of glaucoma in St. Lucia, West Indies. Part I. Prevalence findings. Ophthalmology. 1989;96:1363–8.
Strouthidis NG, Yang H, Reynaud JF, Grimm JL, Gardiner SK, Fortune B, et al. Comparison of clinical and spectral domain optical coherence tomography optic disc margin anatomy. Investig Ophthalmol Vis Sci. 2009;50:4709–18.
Reis AS, Sharpe GP, Yang H, Nicolela MT, Burgoyne CF, Chauhan BC. Optic disc margin anatomy in patients with glaucoma and normal controls with spectral domain optical coherence tomography. Ophthalmology. 2012;119:738–47.
Reis AS, O’Leary N, Yang H, Sharpe GP, Nicolela MT, Burgoyne CF, et al. Influence of clinically invisible, but optical coherence tomography detected, optic disc margin anatomy on neuroretinal rim evaluation. Investig Ophthalmol Vis Sci. 2012;53:1852–60.
Povazay B, Hofer B, Hermann B, Unterhuber A, Morgan JE, Glittenberg C, et al. Minimum distance mapping using three-dimensional optical coherence tomography for glaucoma diagnosis. J Biomed Opt. 2007;12:041204.
Chen TC. Spectral domain optical coherence tomography in glaucoma: qualitative and quantitative analysis of the optic nerve head and retinal nerve fiber layer (an AOS thesis). Trans Am Ophthalmol Soc. 2009;107:254–81.
Strouthidis NG, Fortune B, Yang H, Sigal IA, Burgoyne CF. Longitudinal change detected by spectral domain optical coherence tomography in the optic nerve head and peripapillary retina in experimental glaucoma. Investig Ophthalmol Vis Sci. 2011;52:1206–19.
Chauhan BC, Burgoyne CF. From clinical examination of the optic disc to clinical assessment of the optic nerve head: a paradigm change. Am J Ophthalmol. 2013;156:218–27.e2.
Chauhan BC, O’Leary N, Almobarak FA, Reis ASC, Yang H, Sharpe GP, et al. Enhanced detection of open-angle glaucoma with an anatomically accurate optical coherence tomography-derived neuroretinal rim parameter. Ophthalmology. 2013;120:535–43.
Yamashita T, Sakamoto T, Yoshihara N, Terasaki H, Tanaka M, Kii Y, et al. Peripapillary nerve fiber elevation in young healthy eyes. Investig Ophthalmol Vis Sci. 2016;57:4368–72.
Terasaki H, Yamashita T, Tanaka M, Nakao K, Sakamoto T. Relationship between funduscopic conus and optic disc factors associated with myopia in young healthy eyes. Investig Ophthalmol Vis Sci. 2020;61:40.
Yamashita T, Sakamoto T, Terasaki H, Tanaka M, Kii Y, Nakao K. Quantification of retinal nerve fiber and retinal artery trajectories using second-order polynomial equation and its association with axial length. Investig Ophthalmol Vis Sci. 2014;55:5176–82.
Yamashita T, Sakamoto T, Yoshihara N, Terasaki H, Kii Y, Tanaka M, et al. Circumpapillary course of retinal pigment epithelium can be fit to sine wave and amplitude of sine wave is significantly correlated with ovality ratio of optic disc. PLOS One. 2015;10:e0122191.
Wang YX, Panda-Jonas S, Jonas JB. Optic nerve head anatomy in myopia and glaucoma, including parapapillary zones alpha, beta, gamma and delta: histology and clinical features. Prog Retin Eye Res. 2021;83:100933.
Jonas JB, Fang Y, Weber P, Ohno-Matsui K. Parapapillary gamma and delta zones in high myopia. Retina. 2018;38:931–8.
Terasaki H, Yamashita T, Yoshihara N, Kii Y, Tanaka M, Nakao K, et al. Location of tessellations in ocular fundus and their associations with optic disc tilt, optic disc area, and axial length in young healthy eyes. PLoS One. 2016;11:e0156842.
Jung Y, Park HY, Park CK. Association between corneal deformation amplitude and posterior pole profiles in primary open-angle glaucoma. Ophthalmology. 2016;123:959–64.
Bennett AG, Rudnicka AR, Edgar DF. Improvements on Littmann’s method of determining the size of retinal features by fundus photography. Graefes Arch Clin Exp Ophthalmol. 1994;232:361–7.
Spaide RF, Ohno-Matsui K, Yannuzzi LA. Pathologic myopia. Springer; 2014.
Ohno-Matsui K, Jonas JB. Posterior staphyloma in pathologic myopia. Prog Retin Eye Res. 2019;70:99–109.
Yamashita T, Terasaki H, Tanaka M, Nakao K, Sakamoto T. Relationship between peripapillary choroidal thickness and degree of tessellation in young healthy eyes. Graefes Arch Clin Exp Ophthalmol. 2020;258:1779–85.
Sawada A, Tomidokoro A, Araie M, Iwase A, Yamamoto T, Tajimi Study Group. Refractive errors in an elderly Japanese population: the Tajimi study. Ophthalmology. 2008;115:363–70.e3.
Acknowledgements
We would like to thank Editage (www.editage.com) for English language editing. This work was supported in part by JSPS KAKENHI grant 15H04996. The funders had no role in the study design, data collection and analysis, decision to publish, or manuscript preparation.
Author information
Authors and Affiliations
Contributions
Conception or design of the work: TY. Acquisition, analysis, or interpretation of data: KF, TY, HT, KN, TS. Draughting the work: KF, TY. Revising the work: HT, KN, TS.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics
All the procedures conformed to the tenets of the Declaration of Helsinki. We obtained written informed consent from all participants following an explanation of the procedures used. We obtained official approval for this study from the Ethics Committee of Kagoshima University Hospital and registered it with the University Hospital Medical Network Clinical Trials Registry (the registration number; UMIN000007154).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fujiwara, K., Yamashita, T., Terasaki, H. et al. Quantification of peripapillary nerve fibre elevation and its association with axial length, optic disc tilt, and parapapillary atrophy area in young, healthy eyes. Eye 38, 1112–1117 (2024). https://doi.org/10.1038/s41433-023-02827-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-023-02827-y