Abstract
Purpose
To assess factors associated with failure of intravenous methylprednisolone (IVMP) monotherapy as the first-line treatment for thyroid eye disease (TED) and to identify patients who might benefit from supplementing mycophenolate mofetil (MMF) to IVMP.
Methods
Data for all patients with TED treated with IVMP according to the EUGOGO protocol in our center between 2016–2021 were retrospectively analysed.
Results
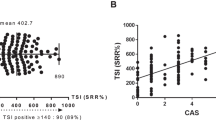
Forty-seven patients (mean age 51.32 ± 14 years, 27 females) were enrolled. The mean time from first reported symptoms to first IVMP treatment was 12.1 ± 5.59 months (range 0–120). The mean clinical activity score (CAS) before treatment and at a mean of 5 and 12.2 weeks after treatment initiation was 6.00, 2.96, and 1.81, respectively (P < 0.01). Twenty-one patients (44.68%) were recommended second-line treatment: nine due to no response or worsening of CAS, six due to partial response, four with good response but early relapse after completion of treatment, and one due to late relapse. Eighteen of those 21 patients received second-line treatment which included rituximab (n = 7), MMF (n = 6), a second course of IVMP (n = 4), and tocilizumab (n = 1). Serum thyroid-stimulating immunoglobulin (TSI) levels were higher in patients who received second-line treatment compared with patients who responded well to first-line IVMP monotherapy at presentation (2135% vs 1159%, P = 0.05) and after completion of first-line treatment (2201% vs. 986%, P = 0.043).
Discussion
TED patients requiring second-line treatment after failed IVMP monotherapy had higher baseline and post-first-line treatment serum TSI levels. Those with elevated TSI may benefit from dual therapy (IVMP and MMF) and require closer monitoring.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 18 print issues and online access
$259.00 per year
only $14.39 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Iyer S, Bahn R. Immunopathogenesis of Graves’ ophthalmopathy: the role of the TSH receptor. Best Pract Res Clin Endocrinol Metab. 2012;26:281–9. https://doi.org/10.1016/j.beem.2011.10.003.
Ponto KA, Kanitz M, Olivo PD, Pitz S, Pfeiffer N, Kahaly GJ. Clinical relevance of thyroid-stimulating immunoglobulins in graves’ ophthalmopathy. Ophthalmology. 2011;118:2279–85. https://doi.org/10.1016/j.ophtha.2011.03.030.
Kahaly GJ. The thyrocyte-fibrocyte link: closing the loop in the pathogenesis of Graves’ disease. J Clin Endocrinol Metab. 2010;95:62–5. https://doi.org/10.1210/jc.2009-2405.
Bahn RS, Dutton CM, Natt N, Joba W, Spitzweg C, Heufelder AE. Thyrotropin receptor expression in Graves’ orbital adipose/connective tissues: potential autoantigen in Graves’ ophthalmopathy. J Clin Endocrinol Metab. 1998;83:998–1002. https://doi.org/10.1210/jcem.83.3.4676.
Bartalena L, Kahaly GJ, Baldeschi L, Dayan CM, Eckstein A, Marcocci C. et al. The 2021 European Group on Graves’ Orbitopathy (EUGOGO) clinical practice guidelines for the medical management of Graves’ orbitopathy. Eur J Endocrinol. 2021;185:G43–67. https://doi.org/10.1530/eje-21-0479.
Kahaly G, Pitz S, Hommel G, Dittmar M. Randomized, single blind trial of intravenous versus oral steroid monotherapy in Graves’ orbitopathy. J Clin Endocrinol Metab. 2005;90:5234–40.
Zang S, Ponto K, Kahaly G. Clinical review: intravenous glucocorticoids for Graves’ orbitopathy: efficacy and morbidity. J Clin Endocrinol Metab. 2011;96:320–32.
van Geest RJ, Sasim IV, Koppeschaar HP, Kalmann R, Stravers SN, Bijlsma WR, et al. Methylprednisolone pulse therapy for patients with moderately severe Graves’ orbitopathy: a prospective, randomized, placebo-controlled study. Eur J Endocrinol. 2008;158:229–37.
Kahaly GJ, Riedl M, König J, Pitz S, Ponto K, Diana T, et al. Mycophenolate plus methylprednisolone versus methylprednisolone alone in active, moderate-to-severe Graves’ orbitopathy (MINGO): a randomised, observer-masked, multicentre trial. Lancet: Diabetes Endocrinol. 2018;6:287–98.
Ahn HY, Lee JK. Intravenous glucocorticoid treatment for Korean graves’ ophthalmopathy patients. J Korean Med Sci. 2020;35:e177. https://doi.org/10.3346/jkms.2020.35.e177.
Orgiazzi J. Adding the immunosuppressant mycophenolate mofetil to medium-dose infusions of methylprednisolone improves the treatment of Graves’ orbitopathy. Clin Thyroidol. 2018;30:10–14.
Bartalena L, Baldeschi L, Dickinson AJ, Eckstein A, Kendall-Taylor P, Marcocci C. et al. Consensus statement of the European Group on Graves’ orbitopathy (EUGOGO) on management of Graves’ orbitopathy. Thyroid. 2008;18:333–46. https://doi.org/10.1089/thy.2007.0315.
Lytton SD, Kahaly GJ. Bioassays for TSH-receptor autoantibodies: an update. Autoimmun Rev. 2010;10:116–22. https://doi.org/10.1016/j.autrev.2010.08.018.
Ko J, Kook KH, Yoon JS, Woo KI, Yang JW. Longitudinal association of thyroid-stimulating immunoglobulin levels with clinical characteristics in thyroid eye disease. BMJ Open. 2022;12:e050337. https://doi.org/10.1136/bmjopen-2021-050337.
Jang SY, Shin DY, Lee EJ, Yoon JS. Clinical characteristics of Graves’ orbitopathy in patients showing discrepancy between levels from TBII assays and TSI bioassay. Clin Endocrinol. 2014;80:591–7. https://doi.org/10.1111/cen.12318.
Shen L, Huang F, Ye L, Zhu W, Zhang X, Wang S. et al. Circulating microRNA predicts insensitivity to glucocorticoid therapy in Graves’ ophthalmopathy. Endocrine. 2015;49:445–56. https://doi.org/10.1007/s12020-014-0487-4.
Ye X, Bo X, Hu X, Cui H, Lu B, Shao J, et al. Efficacy and safety of mycophenolate mofetil in patients with active moderate-to-severe Graves’ orbitopathy. Clin Endocrinol. 2017;86:247–55.
Lee A, Riedl M, Frommer L, Diana T, Kahaly G. Systemic safety analysis of mycophenolate in Graves’ orbitopathy. J Endocrinol Investig. 2020;43:767–77.
Riedl M, Kuhn A, Krämer I, Kolbe E, Kahaly G. Prospective, systematically recorded mycophenolate safety data in Graves’ orbitopathy. J Endocrinol Investig. 2016;39:687–94.
Author information
Authors and Affiliations
Contributions
Conception/design of the research: OZ and OS. Data collection: AR. Analysis/interpretation: OZ and OS. Drafting the manuscript: OS. Revision of the manuscript: OZ, AR, AP, DLP, TCY, RS, NAL, and GJBS. Final approval of the manuscript: OZ, AR, AP, DLP, TCY, RS, NAL, GJBS, and OS.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zloto, O., Rosset, A., Priel, A. et al. Elevated serum thyroid stimulating immunoglobulin linked to failure of first-line intravenous methylprednisolone monotherapy in moderate-to-severe thyroid eye disease. Eye 38, 687–690 (2024). https://doi.org/10.1038/s41433-023-02748-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-023-02748-w