Abstract
Purpose
To evaluate the quality of life (QoL), mental health conditions and corneal morphology in neuropathic corneal pain (NCP) subjects without a significant ocular surface disease.
Methods
A composite questionnaire was administered to 228 consecutive subjects, assessing the pain intensity, duration, and quality using a modified version of the Self-Administered Leeds Assessment of Neuropathic Symptoms and Signs (S-LANSS) and Pain Detect (PD) questionnaires. Subjects diagnosed with possible central NCP and two sub-groups of patients diagnosed with peripheral ocular pain completed an additional battery of mental health questionnaires and were examined by In Vivo Confocal Microscopy (IVCM).
Results
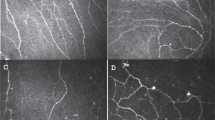
Of the 76 subjects that reported chronic ocular pain (duration >1 month), 53 were classified with probable NCP. Nine subjects without signs that justify the pain and non-responding to topical anaesthesia, were considered affected by central NCP. In these patients, a significant negative correlation was found between the presence pain and the mental component of the QoL (R2 = 0.733), and a positive correlation between the severity of pain the presence post-traumatic stress disorder (R2 = 0.83) and depression (R2 = 0.93). Although neuromas and sprouting had higher frequency in the central NCP group compared the control groups, these differences was not statistically different.
Conclusions
The assessment of ocular pain characteristics using multiple questionnaires and IVCM may help to recognize differences between nociceptive and neuropathic pain. An association between pain intensity and mental health condition may guide the therapeutical choices.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 18 print issues and online access
$259.00 per year
only $14.39 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
Original data are stored in the hospital database along with results of the confocal microscopy findings. All scores are available upon request to the corresponding author.
References
Raja SN, Carr DB, Cohen M, Finnerup NB, Flor H, Gibson S, et al. The revised International Association for the Study of Pain definition of pain: concepts, challenges, and compromises. Pain. 2020;161:1976–82.
Jensen TS, Baron R, Haanpaa M, Kalso E, Loeser JD, Rice ASC, et al. A new definition of neuropathic pain. Pain. 2011;152:2204–5.
Woolf CJ, Mannion RJ. Neuropathic pain: aetiology, symptoms, mechanisms, and management. Lancet. 1999;353:1959–64.
Mills SEE, Nicolson KP, Smith BH. Chronic pain: a review of its epidemiology and associated factors in population-based studies. Br J Anaesth. 2019;123:e273–83.
Rosenthal P, Borsook D. Ocular neuropathic pain. Br J Ophthalmol. 2016;100:128–34.
Rosenthal P, Borsook D. The corneal pain system. Part I: the missing piece of the dry eye puzzle. Ocul Surf. 2012;10:2–14.
Rosenthal P, Borsook D, Moulton EA. Oculofacial pain: corneal nerve damage leading to pain beyond the eye. Investig Ophthalmol Vis Sci. 2016;57:5285–7.
Dieckmann G, Goyal S, Hamrah P. Neuropathic corneal pain: approaches for management. Ophthalmology. 2017;124:S34–47.
Li W, Graham AD, Lin MC. Understanding ocular discomfort and dryness using the pain sensitivity questionnaire. PLoS One. 2016;11:e0154753.
Qazi Y, Hurwitz S, Khan S, Jurkunas UV, Dana R, Hamrah P. Validity and Reliability of a Novel Ocular Pain Assessment Survey (OPAS) in Quantifying and Monitoring Corneal and Ocular Surface Pain. Ophthalmology. 2016;123:1458–68.
Goyal S, Hamrah P. Understanding neuropathic corneal pain-gaps and current therapeutic approaches. Semin Ophthalmol. 2016;31:59–70.
Belmonte C, Aracil A, Acosta MC, Luna C, Gallar J. Nerves and sensations from the eye surface. Ocul Surf. 2004;2:248–53.
Hamrah P, Qazi Y, Shahatit B, Dastjerdi MH, Pavan-Langston D, Jacobs DS, et al. Corneal nerve and epithelial cell alterations in corneal allodynia: an in vivo confocal microscopy case series. Ocul Surf. 2017;15:139–51.
Aggarwal S, Kheirkhah A, Cavalcanti BM, Cruzat A, Colon C, Brown E, et al. Autologous serum tears for treatment of photoallodynia in patients with corneal neuropathy: efficacy and evaluation with in vivo confocal microscopy. Ocul Surf. 2015;13:250–62.
Mehra D, Cohen NK, Galor A. Ocular surface pain: a narrative review. Ophthalmol Ther. 2020;9:1–21.
Dermer H, Hwang J, Mittal R, Cohen AK, Galor A. Corneal sub-basal nerve plexus microneuromas in individuals with and without dry eye. Br J Ophthalmol. 2022;106:616–22.
Ross AR, Al-Aqaba MA, Almaazmi A, Messina M, Nubile M, Mastropasqua L, et al. Clinical and in vivo confocal microscopic features of neuropathic corneal pain. Br J Ophthalmol. 2020;104:768–75.
Farhangi M, Feuer W, Galor A, Bouhassira D, Levitt RC, Sarantopoulos CD, et al. Modification of the Neuropathic Pain Symptom Inventory for use in eye pain (NPSI-Eye). Pain. 2019;160:1541–50.
Stephenson NL, Herman JA. Pain measurement: a comparison using horizontal and vertical visual analogue scales. Appl Nurs Res. 2000;13:157–8.
Bieri D, Reeve RA, Champion DG, Addicoat L, Ziegler JB. The Faces Pain Scale for the self-assessment of the severity of pain experienced by children: development, initial validation, and preliminary investigation for ratio scale properties. Pain. 1990;41:139–50.
Maiani G, Sanavio E. Semantics of pain in Italy: the Italian version of the McGill Pain Questionnaire. Pain. 1985;22:399–405.
Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singap. 1994;23:129–38.
Bennett MI, Smith BH, Torrance N, Potter J. The S-LANSS score for identifying pain of predominantly neuropathic origin: validation for use in clinical and postal research. J Pain. 2005;6:149–58.
Yoon HJ, Kim J, Yoon KC. Treatment response to gabapentin in neuropathic ocular pain associated with dry eye. J Clin Med. 2020;9:3765.
Balanaser M, Carley M, Baron R, Finnerup NB, Moore RA, Rowbotham MC, et al. Combination pharmacotherapy for the treatment of neuropathic pain in adults: systematic review and meta-analysis. Pain. 2023;164:230–51.
Garcia-Lopez C, Gomez-Huertas C, Sanchez-Gonzalez JM, Borroni D, Rodriguez-Calvo-de-Mora M, Romano V, et al. Opioids and ocular surface pathology: a literature review of new treatments horizons. J Clin Med. 2022;11:1424.
Patel S, Mittal R, Felix ER, Sarantopoulos KD, Levitt RC, Galor A. Differential effects of treatment strategies in individuals with chronic ocular surface pain with a neuropathic component. Front Pharm. 2021;12:788524.
Freynhagen R, Baron R, Gockel U, Tolle TR. painDETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin. 2006;22:1911–20.
Wolffsohn JS, Arita R, Chalmers R, Djalilian A, Dogru M, Dumbleton K, et al. TFOS DEWS II Diagnostic Methodology report. Ocul Surf. 2017;15:539–74.
Guerrero-Moreno A, Liang H, Moreau N, Luzu J, Rabut G, Melik Parsadaniantz S, et al. Corneal nerve abnormalities in painful dry eye disease patients. Biomedicines. 2021;9:1424.
Crane AM, Feuer W, Felix ER, Levitt RC, McClellan AL, Sarantopoulos KD, et al. Evidence of central sensitisation in those with dry eye symptoms and neuropathic-like ocular pain complaints: incomplete response to topical anaesthesia and generalised heightened sensitivity to evoked pain. Br J Ophthalmol. 2017;101:1238–43.
Apolone G, Mosconi P. The Italian SF-36 Health Survey: translation, validation and norming. J Clin Epidemiol. 1998;51:1025–36.
Spitzer RL, Kroenke K, Williams JB, Lowe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092–7.
Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6:278–96.
Darnall BD, Sturgeon JA, Cook KF, Taub CJ, Roy A, Burns JW, et al. Development and Validation of a Daily Pain Catastrophizing Scale. J Pain. 2017;18:1139–49.
Monticone M, Baiardi P, Ferrari S, Foti C, Mugnai R, Pillastrini P, et al. Development of the Italian version of the Pain Catastrophising Scale (PCS-I): cross-cultural adaptation, factor analysis, reliability, validity and sensitivity to change. Qual life Res. 2012;21:1045–50.
Blanchard EB, Jones-Alexander J, Buckley TC, Forneris CA. Psychometric properties of the PTSD Checklist (PCL). Behav Res Ther. 1996;34:669–73.
Freedy JR, Steenkamp MM, Magruder KM, Yeager DE, Zoller JS, Hueston WJ, et al. Post-traumatic stress disorder screening test performance in civilian primary care. Fam Pr. 2010;27:615–24.
Chao C, Stapleton F, Badarudin E, Golebiowski B. Ocular surface sensitivity repeatability with Cochet-Bonnet esthesiometer. Optom Vis Sci. 2015;92:183–9.
Oliveira-Soto L, Efron N. Morphology of corneal nerves using confocal microscopy. Cornea. 2001;20:374–84.
Chinnery HR, Rajan R, Jiao H, Wu M, Zhang AC, De Silva MEH, et al. Identification of presumed corneal neuromas and microneuromas using laser-scanning in vivo confocal microscopy: a systematic review. Br J Ophthalmol. 2022;106:765–71.
Rosenthal P, Baran I, Jacobs DS. Corneal pain without stain: is it real? Ocul Surf. 2009;7:28–40.
Mehra D, Mangwani-Mordani S, Acuna K, C Hwang J, R Felix E, Galor A. Long-Term Trigeminal Nerve Stimulation as a Treatment for Ocular Pain. Neuromodulation. 2021;24:1107–14.
Moein H-RDG, Abbouda A, Pondelis N, Jamali A, Salem Z, Hamrah P. In Vivo Confocal Microscopy Demonstrates the Presence of Microneuromas and may Allow Differentiation of Patients with Corneal Neuropathic Pain from Dry Eye Disease. Investig Ophthalmol Vis Sci. 2017;58:2656.
Benitez-Del-Castillo JM, Acosta MC, Wassfi MA, Diaz-Valle D, Gegundez JA, Fernandez C, et al. Relation between corneal innervation with confocal microscopy and corneal sensitivity with noncontact esthesiometry in patients with dry eye. Investig Ophthalmol Vis Sci. 2007;48:173–81.
Spierer O, Felix ER, McClellan AL, Parel JM, Gonzalez A, Feuer WJ, et al. Corneal Mechanical Thresholds Negatively Associate With Dry Eye and Ocular Pain Symptoms. Investig Ophthalmol Vis Sci. 2016;57:617–25.
Ferrari G, Bignami F, Giacomini C, Capitolo E, Comi G, Chaabane L, et al. Ocular surface injury induces inflammation in the brain: in vivo and ex vivo evidence of a corneal-trigeminal axis. Investig Ophthalmol Vis Sci. 2014;55:6289–300.
Belmonte C, Acosta MC, Gallar J. Neural basis of sensation in intact and injured corneas. Exp Eye Res. 2004;78:513–25.
Belmonte C, Nichols JJ, Cox SM, Brock JA, Begley CG, Bereiter DA, et al. TFOS DEWS II pain and sensation report. Ocul Surf. 2017;15:404–37.
Zhou Y, Murrough J, Yu Y, Roy N, Sayegh R, Asbell P, et al. Association Between Depression and Severity of Dry Eye Symptoms, Signs, and Inflammatory Markers in the DREAM Study. JAMA Ophthalmol. 2022;140:392–9.
Wan KH, Chen LJ, Young AL. Depression and anxiety in dry eye disease: a systematic review and meta-analysis. Eye. 2016;30:1558–67.
Cavalli E, Mammana S, Nicoletti F, Bramanti P, Mazzon E. The neuropathic pain: An overview of the current treatment and future therapeutic approaches. Int J Immunopathol Pharm. 2019;33:2058738419838383.
Rapson LM, Wells N, Pepper J, Majid N, Boon H. Acupuncture as a promising treatment for below-level central neuropathic pain: a retrospective study. J Spinal Cord Med. 2003;26:21–6.
Maggioni F, Leonardi A. Does Ocular Neuropathic Pain Deserve an Autonomous Position in the IHS Classification? Clinical and Diagnostic Evidences. Headache. 2017;57:962–3.
Author information
Authors and Affiliations
Contributions
Conception and design: DL, AL, UF, FM. Analysis and interpretation: AL, OMF, UF, FC. Writing the article: AL, OMF, ES. Critical revision of the article: ES, FC, UF, FM, DL. Final approval of the article: AL, OMF, ES, FC, DL, UF, FM. Data Collection: AL, UR, ES, OMF, DL. Provision of materials, patients, or resources: AL, DL, UR. Statistical expertise: FC. Obtaining funding: AL, UF. Literature search: AL, OMF, UF, EL. Administrative, technical or logistic support: DL, FC. Statement about conformity with author information: none.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Leonardi, A., Feuerman, O.M., Salami, E. et al. Coexistence of neuropathic corneal pain, corneal nerve abnormalities, depression, and low quality of life. Eye 38, 499–506 (2024). https://doi.org/10.1038/s41433-023-02710-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-023-02710-w
This article is cited by
-
Reducing the stress of corneal neuropathic pain: ‘Pain without Stain’
Eye (2024)
-
Corneal neuropathic pain: a review to inform clinical practice
Eye (2024)
-
Is there a relationship between the severity of disease in major depressive disorder patients and dry eye disease?
International Ophthalmology (2024)