Abstract
Objective
To investigate relationship of the retinal capillary plexus (RCP) and ganglion cell complex (GCC) with mild cognitive impairment (MCI) and dementia in a community-based study1.
Methods
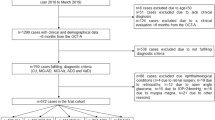
This cross-sectional study incorporated the participants of the Jidong Eye Cohort Study. Optical coherence tomography angiography was performed to obtain RCP vessel density and GCC thickness with detailed segments. The Mini-mental State Examination and Montreal Cognitive Assessment were used to assess cognitive status by professional neuropsychologists. Participants were thus divided into three groups: normal, mild cognitive impairment, and dementia. Multivariable analysis was used to measure relationship of ocular parameters with cognitive impairment.
Results
Of the 2678 participants, the mean age was 44.1 ± 11.7 years. MCI and dementia occurred in 197 (7.4%) and 80 (3%) participants, respectively. Compared to the normal group, the adjusted odds ratio (OR) with the 95% confidence interval was 0.76 (0.65–0.90) for the correlation of lower deep RCP with MCI. We found the following items significantly associated with dementia compared with the normal group: a superficial (OR, 0.68 [0.54–0.86]) and deep (OR, 0.75 [0.57–0.99]) RCP, as well as the GCC (OR, 0.68 [0.54–0.85]). Compared to the MCI group, those with dementia had decreased GCC (OR, 0.75 [0.58–0.97]).
Conclusions
Decreased deep RCP density was associated with MCI. Decreased superficial and deep RCP and the thin GCC were correlated with dementia. These implied that the retinal microvasculature may develop into a promising non-invasive imaging marker to predict severity of cognitive impairment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 18 print issues and online access
$259.00 per year
only $14.39 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Data are available upon reasonable request.
References
Nichols E, Szoeke CEI, Vollset SE, Abbasi N, Abd-Allah F, Abdela J, et al. Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:88–106.
Nichols E, Steinmetz JD, Vollset SE, Fukutaki K, Chalek J, Abd-Allah F, et al. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. Lancet Public Health. 2022;7:e105–25.
Gauthier S, Reisberg B, Zaudig M, Petersen RC, Ritchie K, Broich K, et al. Mild cognitive impairment. Lancet. 2006;367:1262–70.
Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging‐Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:280–92.
Porsteinsson AP, Isaacson RS, Knox S, Sabbagh MN, Rubino I. Diagnosis of early Alzheimer’s disease: clinical practice in 2021. J Prev Alzheimers Dis. 2021;8:371–86.
Grossman I, Lutz MW, Crenshaw DG, Saunders AM, Burns DK, Roses AD. Alzheimer’s disease: diagnostics, prognostics and the road to prevention. EPMA J. 2010;1:293–303.
Veitch DP, Weiner MW, Aisen PS, Beckett LA, Cairns NJ, Green RC, et al. Understanding disease progression and improving Alzheimer’s disease clinical trials: recent highlights from the Alzheimer’s Disease Neuroimaging Initiative. Alzheimers Dement. 2019;15:106–52.
Lima AA, Mridha MF, Das SC, Kabir MM, Islam MDR, Watanobe Y. A comprehensive survey on the detection, classification, and challenges of neurological disorders. Biology. 2022;11:469.
Shi H, Koronyo Y, Rentsendorj A, Fuchs DT, Sheyn J, Black KL, et al. Retinal vasculopathy in Alzheimer’s disease. Front Neurosci. 2021;15:731614.
Cabrera DeBuc D, Somfai GM, Koller A. Retinal microvascular network alterations: potential biomarkers of cerebrovascular and neural diseases. Am J Physiol Heart Circ Physiol. 2017;312:H201–12.
Chiquita S, Rodrigues-Neves AC, Baptista FI, Carecho R, Moreira PI, Castelo-Branco M, et al. The Retina as a window or mirror of the brain changes detected in Alzheimer’s disease: critical aspects to unravel. Mol Neurobiol. 2019;56:5416–35.
Feke GT, Hyman BT, Stern RA, Pasquale LR. Retinal blood flow in mild cognitive impairment and Alzheimer’s disease. Alzheimers Dement. 2015;1:144–51.
Berisha F, Feke GT, Trempe CL, McMeel JW, Schepens CL. Retinal abnormalities in early Alzheimer’s disease. Invest Ophthalmol Vis Sci. 2007;48:2285–9.
Stefánsson E, Olafsdottir OB, Eliasdottir TS, Vehmeijer W, Einarsdottir AB, Bek T, et al. Retinal oximetry: Metabolic imaging for diseases of the retina and brain. Prog Retin Eye Res. 2019;70:1–22.
Szegedi S, Dal-Bianco P, Stögmann E, Traub-Weidinger T, Rainer M, Masching A, et al. Anatomical and functional changes in the retina in patients with Alzheimer’s disease and mild cognitive impairment. Acta Ophthalmol. 2020;98:e914–21.
Bock M, Brandt AU, Dörr J, Pfueller CF, Ohlraun S, Zipp F, et al. Time domain and spectral domain optical coherence tomography in multiple sclerosis: a comparative cross-sectional study. Mult Scler. 2010;16:893–6.
Kashani AH, Chen CL, Gahm JK, Zheng F, Richter GM, Rosenfeld PJ, et al. Optical coherence tomography angiography: a comprehensive review of current methods and clinical applications. Prog Retin Eye Res. 2017;60:66–100.
Wang L, Murphy O, Caldito NG, Calabresi PA, Saidha S. Emerging applications of Optical Coherence Tomography Angiography (OCTA) in neurological research. Eye Vis. 2018;5:11.
Tey KY, Teo K, Tan ACS, Devarajan K, Tan B, Tan J, et al. Optical coherence tomography angiography in diabetic retinopathy: a review of current applications. Eye Vis. 2019;6:37.
Gao L, Liu Y, Li X, Bai Q, Liu P. Abnormal retinal nerve fiber layer thickness and macula lutea in patients with mild cognitive impairment and Alzheimer’s disease. Arch Gerontol Geriatr. 2015;60:162–7.
Cheung CY, Ong YT, Hilal S, Ikram MK, Low S, Ong YL, et al. Retinal ganglion cell analysis using high-definition optical coherence tomography in patients with mild cognitive impairment and Alzheimer’s disease. J Alzheimers Dis. 2015;45:45–56.
Ferrari L, Huang SC, Magnani G, Ambrosi A, Comi G, Leocani L. Optical coherence tomography reveals retinal neuroaxonal thinning in frontotemporal dementia as in Alzheimer’s disease. J Alzheimers Dis. 2017;56:1101–7.
Gameiro GR, Jiang H, Liu Y, Deng Y, Sun X, Nascentes B, et al. Retinal tissue hypoperfusion in patients with clinical Alzheimer’s disease. Eye Vis. 2018;5:21.
Yoon SP, Grewal DS, Thompson AC, Polascik BW, Dunn C, Burke JR, et al. Retinal microvascular and neurodegenerative changes in Alzheimer’s disease and mild cognitive impairment compared with control participants. Ophthalmol Retina. 2019;3:489–99.
Yan Y, Wu X, Wang X, Geng Z, Wang L, Xiao G, et al. The retinal vessel density can reflect cognitive function in patients with Alzheimer’s disease: evidence from optical coherence tomography angiography. J Alzheimers Dis. 2021;79:1307–16.
Yang K, Cui L, Chen X, Yang C, Zheng J, Zhu X, et al. Decreased vessel density in retinal capillary plexus and thinner ganglion cell complex associated with cognitive impairment. Front Aging Neurosci. 2022;14:872466.
Chua J, Hu Q, Ke M, Tan B, Hong J, Yao X, et al. Retinal microvasculature dysfunction is associated with Alzheimer’s disease and mild cognitive impairment. Alzheimers Res Ther. 2020;12:161.
Jiang H, Wei Y, Shi Y, Wright CB, Sun X, Gregori G, et al. Altered macular microvasculature in mild cognitive impairment and Alzheimer disease. J Neuro-Ophthalmol. 2018;38:292–8.
Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Colin I. et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment: MOCA: a brief screening tool for MCI. J Am Geriatr Soc. 2005;53:695–9.
Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.”. J Psychiatr Res. 1975;12:189–98.
Yang K, Cui L, Zhang G, Wang X, Zhu X, Xiao Y, et al. The Jidong Eye Cohort Study: objectives, design, and baseline characteristics. Eye Vis. 2020;7:58.
Grading diabetic retinopathy from stereoscopic color fundus photographs-an extension of the modified Airlie House classification. ETDRS report number 10. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991;98(5 Suppl):786–806.
Zawadzki RJ, Capps AG, Kim DY, Panorgias A, Stevenson SB, Hamann B, et al. Progress on developing adaptive optics-optical coherence tomography for in vivo retinal imaging: monitoring and correction of eye motion artifacts. IEEE J Sel Top Quantum Electron. 2014;20:7100912.
Ito Y, Sasaki M, Takahashi H, Nozaki S, Matsuguma S, Motomura K, et al. Quantitative assessment of the retina using OCT and associations with cognitive function. Ophthalmology. 2020;127:107–18.
Liew TM, Feng L, Gao Q, Ng TP, Yap P. Diagnostic utility of Montreal Cognitive Assessment in the Fifth Edition of Diagnostic and Statistical Manual of Mental Disorders: major and mild neurocognitive disorders. J Am Med Dir Assoc. 2015;16:144–8.
Dong Y, Lee WY, Basri NA, Collinson SL, Merchant RA, Venketasubramanian N, et al. The Montreal Cognitive Assessment is superior to the Mini-Mental State Examination in detecting patients at higher risk of dementia. Int Psychogeriatr. 2012;24:1749–55.
Larner AJ. Screening utility of the Montreal Cognitive Assessment (MoCA): in place of-or as well as-the MMSE? Int Psychogeriatr. 2012;24:391–6.
Clarke M, Jagger C, Anderson J, Battcock T, Kelly F, Stern MC. The prevalence of dementia in a total population: a comparison of two screening instruments. Age Ageing. 1991;20:396–403.
Chun CT, Seward K, Patterson A, Melton A, MacDonald-Wicks L. Evaluation of available cognitive tools used to measure mild cognitive decline: a scoping review. Nutrients. 2021;13:3974.
The Lancet Public Health. Will dementia hamper healthy ageing? Lancet Public Health. 2022;7:e93.
You QS, Chan JCH, Ng ALK, Choy BKN, Shih KC, Cheung JJC, et al. Macular vessel density measured with optical coherence tomography angiography and its associations in a large population-based study. Investig Ophthalmol Vis Sci. 2019;60:4830–7.
Ward DD, Mauschitz MM, Bönniger MM, Merten N, Finger RP, Breteler MMB. Association of retinal layer measurements and adult cognitive function: a population-based study. Neurology. 2020;95:e1144–52.
Wu J, Zhang X, Azhati G, Li T, Xu G, Liu F. Retinal microvascular attenuation in mental cognitive impairment and Alzheimer’s disease by optical coherence tomography angiography. Acta Ophthalmol. 2020;98:e781–7.
Koronyo-Hamaoui M, Koronyo Y, Ljubimov AV, Miller CA, Ko MK, Black KL, et al. Identification of amyloid plaques in retinas from Alzheimer’s patients and noninvasive in vivo optical imaging of retinal plaques in a mouse model. NeuroImage. 2011;54:S204–17.
Koronyo Y, Biggs D, Barron E, Boyer DS, Pearlman JA, Au WJ, et al. Retinal amyloid pathology and proof-of-concept imaging trial in Alzheimer’s disease. JCI Insight. 2017;2:93621.
Guo L, Ravindran N, Shamsher E, Hill D, Cordeiro MF. Retinal Changes in Transgenic Mouse Models of Alzheimer’s Disease. Curr Alzheimer Res. 2021;18:89–102.
Shi H, Koronyo Y, Rentsendorj A, Regis GC, Sheyn J, Fuchs DT, et al. Identification of early pericyte loss and vascular amyloidosis in Alzheimer’s disease retina. Acta Neuropathol. 2020;139:813–36.
Shi H, Koronyo Y, Fuchs DT, Sheyn J, Wawrowsky K, Lahiri S, et al. Retinal capillary degeneration and blood-retinal barrier disruption in murine models of Alzheimer’s disease. Acta Neuropathol Commun. 2020;8:202.
Montagne A, Nation DA, Sagare AP, Barisano G, Sweeney MD, Chakhoyan A, et al. APOE4 leads to blood-brain barrier dysfunction predicting cognitive decline. Nature. 2020;581:71–6.
Ruitenberg A, den Heijer T, Bakker SLM, van Swieten JC, Koudstaal PJ, Hofman A, et al. Cerebral hypoperfusion and clinical onset of dementia: the Rotterdam Study. Ann Neurol. 2005;57:789–94.
Caprara C, Thiersch M, Lange C, Joly S, Samardzija M, Grimm C. HIF1A is essential for the development of the intermediate plexus of the retinal vasculature. Invest Ophthalmol Vis Sci. 2011;52:2109–17.
Yu DY, Cringle SJ. Oxygen distribution and consumption within the retina in vascularised and avascular retinas and in animal models of retinal disease. Prog Retin Eye Res. 2001;20:175–208.
Yu DY, Cringle SJ, Su EN. Intraretinal oxygen distribution in the monkey retina and the response to systemic hyperoxia. Invest Ophthalmol Vis Sci. 2005;46:4728–33.
Campbell JP, Zhang M, Hwang TS, Bailey ST, Wilson DJ, Jia Y, et al. Detailed vascular anatomy of the human retina by projection-resolved optical coherence tomography angiography. Sci Rep. 2017;7:42201.
Wareham LK, Calkins DJ. The neurovascular unit in glaucomatous neurodegeneration. Front Cell Dev Biol. 2020;8:452.
Hormel TT, Jia Y, Jian Y, Hwang TS, Bailey ST, Pennesi ME, et al. Plexus-specific retinal vascular anatomy and pathologies as seen by projection-resolved optical coherence tomographic angiography. Prog Retin Eye Res. 2021 Jan;80:100878.
Merten N, Paulsen AJ, Pinto AA, Chen Y, Dillard LK, Fischer ME, et al. Macular ganglion cell-inner plexiform layer as a marker of cognitive and sensory function in midlife. J Gerontol A Biol Sci Med Sci. 2020;75:e42–8.
Zhang Y, Wang Y, Shi C, Shen M, Lu F. Advances in retina imaging as potential biomarkers for early diagnosis of Alzheimer’s disease. Transl Neurodegener. 2021;10:6.
Liu S, Ong YT, Hilal S, Loke YM, Wong TY, Chen CLH, et al. The association between retinal neuronal layer and brain structure is disrupted in patients with cognitive impairment and Alzheimer’s disease. J Alzheimers Dis. 2016;54:585–95.
Cheung CY, Chan VTT, Mok VC, Chen C, Wong TY. Potential retinal biomarkers for dementia: what is new? Curr Opin Neurol. 2019;32:82–91.
Querques G, Borrelli E, Sacconi R, De Vitis L, Leocani L, Santangelo R, et al. Functional and morphological changes of the retinal vessels in Alzheimer’s disease and mild cognitive impairment. Sci Rep. 2019;9:63.
den Haan J, van de Kreeke JA, van Berckel BN, Barkhof F, Teunissen CE, Scheltens P, et al. Is retinal vasculature a biomarker in amyloid proven Alzheimer’s disease? Alzheimers Dement. 2019;11:383–91.
van de Kreeke JA, Nguyen HT, Konijnenberg E, Tomassen J, den Braber A, ten Kate M, et al. Optical coherence tomography angiography in preclinical Alzheimer’s disease. Br J Ophthalmol. 2020;104:157–61.
Funding
This work was supported by the National Natural Science Foundation of China (81900903, 82271047); Zhejiang Provincial Natural Science Foundation of China (LTGY23H120002, LY22H120007), National Key R&D Program of China (2020YFC2008200, 2019YFC0840708); Research Program of Wenzhou Medical University (XY2022010). The funding or sponsor institution had no role in designing or conducting this research.
Author information
Authors and Affiliations
Contributions
FL, LC, and ML had organized and theorized the study and explicated the data. CL, KY, KS, YX, and BS play an important part in the obtainment of data. CL and KY analyzed the data. CL, XZ, and YJ drafted the manuscript. FL, LC, and ML modified the manuscript for academic content.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, C., Zhu, X., Yang, K. et al. Relationship of retinal capillary plexus and ganglion cell complex with mild cognitive impairment and dementia. Eye 37, 3743–3750 (2023). https://doi.org/10.1038/s41433-023-02592-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-023-02592-y